当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Establishing a High-Yielding Cell-Free Protein Synthesis Platform Derived from Vibrio natriegens
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-08-14 00:00:00 , DOI: 10.1021/acssynbio.8b00252 Benjamin J. Des Soye , Samuel R. Davidson , Matthew T. Weinstock 1 , Daniel G. Gibson 1 , Michael C. Jewett
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-08-14 00:00:00 , DOI: 10.1021/acssynbio.8b00252 Benjamin J. Des Soye , Samuel R. Davidson , Matthew T. Weinstock 1 , Daniel G. Gibson 1 , Michael C. Jewett
Affiliation
|
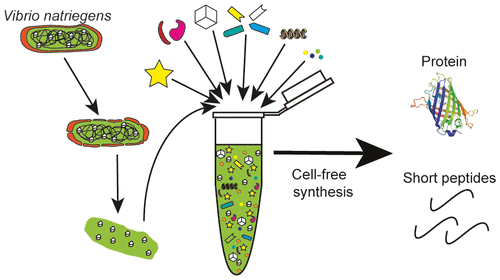
A new wave of interest in cell-free protein synthesis (CFPS) systems has shown their utility for producing proteins at high titers, establishing genetic regulatory element libraries (e.g., promoters, ribosome binding sites) in nonmodel organisms, optimizing biosynthetic pathways before implementation in cells, and sensing biomarkers for diagnostic applications. Unfortunately, most previous efforts have focused on a select few model systems, such as Escherichia coli. Broadening the spectrum of organisms used for CFPS promises to better mimic host cell processes in prototyping applications and open up new areas of research. Here, we describe the development and characterization of a facile CFPS platform based on lysates derived from the fast-growing bacterium Vibrio natriegens, which is an emerging host organism for biotechnology. We demonstrate robust preparation of highly active extracts using sonication, without specialized and costly equipment. After optimizing the extract preparation procedure and cell-free reaction conditions, we show synthesis of 1.6 ± 0.05 g/L of superfolder green fluorescent protein in batch mode CFPS, making it competitive with existing E. coli CFPS platforms. To showcase the flexibility of the system, we demonstrate that it can be lyophilized and retain biosynthesis capability, that it is capable of producing antimicrobial peptides, and that our extract preparation procedure can be coupled with the recently described Vmax Express strain in a one-pot system. Finally, to further increase system productivity, we explore a knockout library in which putative negative effectors of CFPS are genetically removed from the source strain. Our V. natriegens-derived CFPS platform is versatile and simple to prepare and use. We expect it will facilitate expansion of CFPS systems into new laboratories and fields for compelling applications in synthetic biology.
中文翻译:

建立源自钠弧菌的高产无细胞蛋白质合成平台
无细胞蛋白质合成(CFPS)系统中的新一波热潮显示了其在高滴度生产蛋白质,在非模型生物中建立遗传调控元件文库(例如启动子,核糖体结合位点),优化生物合成途径之前的实用性。细胞,以及用于诊断应用的传感生物标记。不幸的是,以前的大多数努力都集中在少数几个模型系统上,例如大肠杆菌。扩大用于CFPS的生物的范围有望在原型应用中更好地模拟宿主细胞过程,并开拓新的研究领域。在这里,我们描述了基于快速增长的细菌衍生的裂解物的便捷CFPS平台的开发和表征弧菌弧菌是一种新兴的生物技术宿主生物。我们展示了使用超声处理高活性提取物的强大制备方法,而无需使用专门且昂贵的设备。优化提取物的制备程序和无细胞反应条件后,我们显示了以分批模式CFPS合成1.6±0.05 g / L的超级文件夹绿色荧光蛋白,使其与现有的大肠杆菌具有竞争力CFPS平台。为了展示该系统的灵活性,我们证明了它可以冻干并保留生物合成能力,能够产生抗微生物肽,并且我们的提取物制备过程可以与最近描述的Vmax Express菌株结合在一锅中系统。最后,为了进一步提高系统生产率,我们探索了一个敲除文库,其中从源菌株中遗传去除了CFPS的推定负效应子。我们的源自V. natriegens的CFPS平台用途广泛,易于制备和使用。我们希望它将促进CFPS系统扩展到新的实验室和领域,以在合成生物学中引人注目的应用。
更新日期:2018-08-14
中文翻译:

建立源自钠弧菌的高产无细胞蛋白质合成平台
无细胞蛋白质合成(CFPS)系统中的新一波热潮显示了其在高滴度生产蛋白质,在非模型生物中建立遗传调控元件文库(例如启动子,核糖体结合位点),优化生物合成途径之前的实用性。细胞,以及用于诊断应用的传感生物标记。不幸的是,以前的大多数努力都集中在少数几个模型系统上,例如大肠杆菌。扩大用于CFPS的生物的范围有望在原型应用中更好地模拟宿主细胞过程,并开拓新的研究领域。在这里,我们描述了基于快速增长的细菌衍生的裂解物的便捷CFPS平台的开发和表征弧菌弧菌是一种新兴的生物技术宿主生物。我们展示了使用超声处理高活性提取物的强大制备方法,而无需使用专门且昂贵的设备。优化提取物的制备程序和无细胞反应条件后,我们显示了以分批模式CFPS合成1.6±0.05 g / L的超级文件夹绿色荧光蛋白,使其与现有的大肠杆菌具有竞争力CFPS平台。为了展示该系统的灵活性,我们证明了它可以冻干并保留生物合成能力,能够产生抗微生物肽,并且我们的提取物制备过程可以与最近描述的Vmax Express菌株结合在一锅中系统。最后,为了进一步提高系统生产率,我们探索了一个敲除文库,其中从源菌株中遗传去除了CFPS的推定负效应子。我们的源自V. natriegens的CFPS平台用途广泛,易于制备和使用。我们希望它将促进CFPS系统扩展到新的实验室和领域,以在合成生物学中引人注目的应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号