当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrocatalytic Study of the Thin Metallopolymer Film of [2,2′‐{1,2‐Ethanediylbis[Nitrilo(1E)‐1‐Ethyl‐1‐Ylidene]}Diphenolate]‐Nickel(II) for Ethanol Electrooxidation
ChemElectroChem ( IF 4 ) Pub Date : 2018-08-28 , DOI: 10.1002/celc.201800532 André Olean-Oliveira 1 , Camila F. Pereira 1 , Diego N. David-Parra 1 , Marcos F. S. Teixeira 1
ChemElectroChem ( IF 4 ) Pub Date : 2018-08-28 , DOI: 10.1002/celc.201800532 André Olean-Oliveira 1 , Camila F. Pereira 1 , Diego N. David-Parra 1 , Marcos F. S. Teixeira 1
Affiliation
|
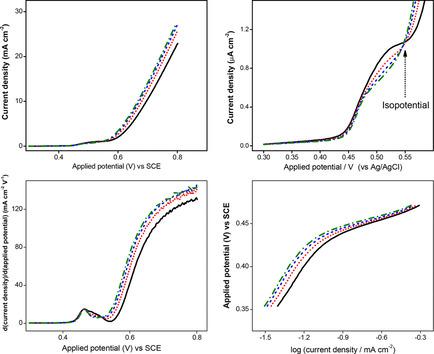
The present paper describes the electrochemical activity of a Ni(II)‐Schiff base metallopolymer electrode for the oxidation of ethanol in alkaline media. The scanning electron microscope results show that the metallopolymer at the glassy carbon surface has a nanoflake‐like lamellar structure, and there is no molecular structural change after treatment with NaOH. Voltammetry measurements indicate that the metallopolymer acts as efficient material for the electrocatalytic oxidation of ethanol, where a high‐valent nickel(IV) was revealed as the reactive intermediate during the anodic scan. An isopotential point can be observed in rotating disk electrode voltammetry for ethanol oxidation. The electrode surface may be considered to consist of two independent electrochemical regions, one corresponding to the ethanol electro‐oxidation by nickel(IV) and the second corresponding to water oxidation. The Tafel slopes were found to be 246 mV dec−1 at low overpotentials and 44 mV dec−1 at high overpotentials, which suggest that the first electron transfer step is the rate controlling step. The specific activity of the metallopolymer‐modified electrode for the ethanol electro‐oxidation reaction was 6.66 mA cm−2 for 0.130 μmol cm−2 of electroactive species of metallopolymer at 0.6 V vs. SCE.
中文翻译:

[2,2'-{1,2-乙二基双[Nitrilo(1E)-1-乙基-1-亚烷基]}二酚盐]-镍(II)的金属聚合物薄膜的电催化乙醇化学氧化研究
本文描述了Ni(II)-Schiff碱金属聚合物电极在碱性介质中氧化乙醇的电化学活性。扫描电子显微镜结果表明,玻璃碳表面的金属聚合物具有纳米片状的层状结构,经NaOH处理后分子结构没有变化。伏安法测量表明,金属聚合物是乙醇电催化氧化的有效材料,其中高价镍(IV)被揭示为阳极扫描过程中的反应性中间体。在乙醇乙醇氧化转盘电极伏安法中可以观察到一个等电位点。可以认为电极表面由两个独立的电化学区域组成,一种对应于镍(IV)对乙醇的电氧化,另一种对应于水的氧化。发现Tafel坡度为246 mV dec-1在低超电势和44毫伏癸-1在高过电位,这表明,第一电子转移步骤是速率控制步骤。在0.6 V vs. SCE下,对于0.130μmolcm -2的金属聚合物电活性物质,金属聚合物修饰的电极对乙醇的电氧化反应的比活度为6.66 mA cm -2。
更新日期:2018-08-28
中文翻译:

[2,2'-{1,2-乙二基双[Nitrilo(1E)-1-乙基-1-亚烷基]}二酚盐]-镍(II)的金属聚合物薄膜的电催化乙醇化学氧化研究
本文描述了Ni(II)-Schiff碱金属聚合物电极在碱性介质中氧化乙醇的电化学活性。扫描电子显微镜结果表明,玻璃碳表面的金属聚合物具有纳米片状的层状结构,经NaOH处理后分子结构没有变化。伏安法测量表明,金属聚合物是乙醇电催化氧化的有效材料,其中高价镍(IV)被揭示为阳极扫描过程中的反应性中间体。在乙醇乙醇氧化转盘电极伏安法中可以观察到一个等电位点。可以认为电极表面由两个独立的电化学区域组成,一种对应于镍(IV)对乙醇的电氧化,另一种对应于水的氧化。发现Tafel坡度为246 mV dec-1在低超电势和44毫伏癸-1在高过电位,这表明,第一电子转移步骤是速率控制步骤。在0.6 V vs. SCE下,对于0.130μmolcm -2的金属聚合物电活性物质,金属聚合物修饰的电极对乙醇的电氧化反应的比活度为6.66 mA cm -2。

























 京公网安备 11010802027423号
京公网安备 11010802027423号