当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Radiotherapy-Controllable Chemotherapy from Reactive Oxygen Species-Responsive Polymeric Nanoparticles for Effective Local Dual Modality Treatment of Malignant Tumors
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1021/acs.biomac.8b00942 Te-I Liu,Ying-Chieh Yang,Wen-Hsuan Chiang,Chun-Kai Hung,Yuan-Chung Tsai,Chi-Shiun Chiang,Chun-Liang Lo,Hsin-Cheng Chiu
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1021/acs.biomac.8b00942 Te-I Liu,Ying-Chieh Yang,Wen-Hsuan Chiang,Chun-Kai Hung,Yuan-Chung Tsai,Chi-Shiun Chiang,Chun-Liang Lo,Hsin-Cheng Chiu
|
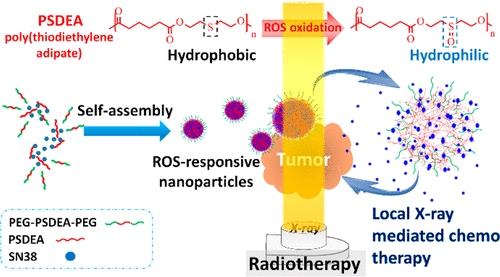
Radiotherapy is one of the general approaches to deal with malignant solid tumors in clinical treatment. To improve therapeutic efficacy, chemotherapy is frequently adopted as the adjuvant treatment in combination with radiotherapy. In this work, a reactive oxygen species (ROS)-responsive nanoparticle (NP) drug delivery system was developed to synergistically enhance the antitumor efficacy of radiotherapy by local ROS-activated chemotherapy, taking advantages of the enhanced concentration of reactive oxygen species (ROS) in tumor during X-ray irradiation and/or reoxygenation after X-ray irradiation. The ROS-responsive polymers, poly(thiodiethylene adipate) (PSDEA) and PEG–PSDEA–PEG, were synthesized and employed as the major components assembling in aqueous phase into polymer NPs in which an anticancer camptothecin analogue, SN38, was encapsulated. The drug-loaded NPs underwent structural change including swelling and partial dissociation in response to the ROS activation by virtue of the oxidation of the nonpolar sulfide residues in NPs into the polar sulfoxide units, thus leading to significant drug unloading. The in vitro performance of the chemotherapy from the X-ray irradiation preactivated NPs against BNL 1MEA.7R.1 murine carcinoma cells showed comparable cytotoxicity to free drug and appreciably enhanced effect on killing cancer cells while the X-ray irradiation being incorporated into the treatment. The in vivo tumor growth was fully inhibited with the mice receiving the local dual modality treatment of X-ray irradiation together with SN38-loaded NPs administered by intratumoral injection. The comparable efficacy of the local combinational treatment of X-ray irradiation with SN38-loaded NPs to free SN38/irradiation dual treatment corroborated the effectiveness of ROS-mediated drug release from the irradiated NPs at tumor site. The IHC examination of tumor tissues confirmed the significant reduction of VEGFA and CD31 expression with the tumor receiving the local dual treatment developed in this work, thus accounting for the absence of tumor regrowth compared to other single modality treatment.
中文翻译:

活性氧反应性聚合物纳米粒子的放射疗法可控化学疗法对恶性肿瘤的有效局部双重治疗。
放射疗法是临床治疗恶性实体瘤的通用方法之一。为了提高治疗效果,化学疗法通常与放射疗法结合用作辅助治疗。在这项工作中,开发了一种活性氧(ROS)反应性纳米颗粒(NP)药物递送系统,以利用活性氧(ROS)浓度增加的优势,协同增强局部ROS激活的化学疗法对放射疗法的抗肿瘤功效。 X射线照射和/或X射线照射后的复氧期间肿瘤中的肿瘤。合成了ROS响应性聚合物,聚己二酸二硫代己二酸酯(PSDEA)和PEG-PSDEA-PEG,并将其作为主要成分在水相中组装成聚合物NP,并封装了抗癌的喜树碱类似物SN38。由于NPs中的非极性硫化物残基被氧化成极性亚砜单元,响应ROS激活,载有药物的NPs发生结构变化,包括溶胀和部分解离。X射线照射预激活的NPs对BNL 1MEA.7R.1鼠癌细胞的化学疗法的体外表现出与游离药物相当的细胞毒性,并且在将X射线照射纳入治疗后,其杀伤癌细胞的作用明显增强。接受X射线照射的局部双重形态治疗的小鼠以及通过肿瘤内注射施用的SN38加载的NP均完全抑制了体内肿瘤的生长。X线照射与SN38 / NP的局部联合治疗与游离SN38 /照射双重治疗的可比疗效证实了ROS介导的从肿瘤部位的NP释放药物介导的药物的有效性。肿瘤组织的IHC检查证实,接受这项工作中开发的局部双重治疗的肿瘤使VEGFA和CD31表达显着降低,因此与其他单一模式治疗相比,无肿瘤再生长。
更新日期:2018-07-25
中文翻译:

活性氧反应性聚合物纳米粒子的放射疗法可控化学疗法对恶性肿瘤的有效局部双重治疗。
放射疗法是临床治疗恶性实体瘤的通用方法之一。为了提高治疗效果,化学疗法通常与放射疗法结合用作辅助治疗。在这项工作中,开发了一种活性氧(ROS)反应性纳米颗粒(NP)药物递送系统,以利用活性氧(ROS)浓度增加的优势,协同增强局部ROS激活的化学疗法对放射疗法的抗肿瘤功效。 X射线照射和/或X射线照射后的复氧期间肿瘤中的肿瘤。合成了ROS响应性聚合物,聚己二酸二硫代己二酸酯(PSDEA)和PEG-PSDEA-PEG,并将其作为主要成分在水相中组装成聚合物NP,并封装了抗癌的喜树碱类似物SN38。由于NPs中的非极性硫化物残基被氧化成极性亚砜单元,响应ROS激活,载有药物的NPs发生结构变化,包括溶胀和部分解离。X射线照射预激活的NPs对BNL 1MEA.7R.1鼠癌细胞的化学疗法的体外表现出与游离药物相当的细胞毒性,并且在将X射线照射纳入治疗后,其杀伤癌细胞的作用明显增强。接受X射线照射的局部双重形态治疗的小鼠以及通过肿瘤内注射施用的SN38加载的NP均完全抑制了体内肿瘤的生长。X线照射与SN38 / NP的局部联合治疗与游离SN38 /照射双重治疗的可比疗效证实了ROS介导的从肿瘤部位的NP释放药物介导的药物的有效性。肿瘤组织的IHC检查证实,接受这项工作中开发的局部双重治疗的肿瘤使VEGFA和CD31表达显着降低,因此与其他单一模式治疗相比,无肿瘤再生长。

























 京公网安备 11010802027423号
京公网安备 11010802027423号