Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-08-07 , DOI: 10.1016/j.bioorg.2018.08.008 Satya Kumar Avula , Ajmal Khan , Najeeb Ur Rehman , Muhammad U. Anwar , Zahra Al-Abri , Abdul Wadood , Muhammad Riaz , Rene Csuk , Ahmed Al-Harrasi
|
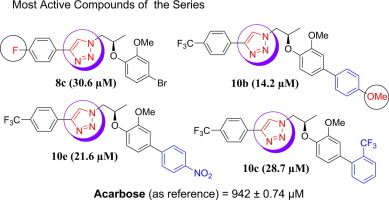
Inhibition of α-glucosidase is an effective strategy for controlling the post-prandial hyperglycemia in diabetic patients. For the identification of new inhibitors of this enzyme, a series of new (R)-1-(2-(4-bromo-2-methoxyphenoxy) propyl)-4-(4-(trifluoromethyl) phenyl)-1H-1,2,3-triazole derivatives were synthesized (8a–d and 10a–e). The structures were confirmed by NMR, mass spectrometry and, in case of compound 8a, by single crystal X-ray crystallography. The α-glucosidase inhibitory activities were investigated in vitro. Most derivatives exhibited significant inhibitory activity against α-glucosidase enzyme. Their structure-activity relationship and molecular docking studies were performed to elucidate the active pharmacophore against this enzyme. Compound 10b was the most active analogue with IC50 value of 14.2 µM, while compound 6 was found to be the least active having 218.1 µM. A preliminary structure-activity relationship suggested that the presence of 1H-1,2,3-triazole ring in 1H-1,2,3-triazole derivatives is responsible for this activity and can be used as anti-diabetic drugs. The molecular docking studies of all active compounds were performed, in order to understand the mode of binding interaction and the energy of this class of compounds.
中文翻译:

1 H -1,2,3-三唑衍生物作为新型α-葡萄糖苷酶抑制剂的合成及其分子对接研究
抑制α-葡萄糖苷酶是控制糖尿病患者餐后高血糖的有效策略。为了鉴定该酶的新抑制剂,一系列新的(R)-1-(2-(4-溴-2-甲氧基苯氧基)丙基)-4-(4-(三氟甲基)苯基)-1 H -1合成了2,2,3-三唑衍生物(8a–d和10a–e)。通过NMR,质谱以及在化合物8a的情况下通过单晶X射线晶体学确认结构。的α葡萄糖苷酶抑制活性进行了研究体外。大多数衍生物对α表现出显着的抑制活性-葡糖苷酶。他们进行了结构-活性关系和分子对接研究,以阐明针对该酶的活性药效团。化合物10b是活性最高的类似物,IC 50值为14.2 µM,而化合物6是活性最低的类似物,为218.1 µM。初步的结构活性关系表明,在1 H -1,2,3-三唑衍生物中存在1 H -1,2,3-三唑环是造成这种活性的原因,可以用作抗糖尿病药。为了了解结合相互作用的方式和这类化合物的能量,对所有活性化合物进行了分子对接研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号