当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
De novo coiled-coil peptides as scaffolds for disrupting protein-protein interactions.
Chemical Science ( IF 8.4 ) Pub Date : 2018-08-07 , DOI: 10.1039/c8sc02643b Jordan M Fletcher 1 , Katherine A Horner 2, 3 , Gail J Bartlett 1 , Guto G Rhys 1 , Andrew J Wilson 2, 3 , Derek N Woolfson 1, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2018-08-07 , DOI: 10.1039/c8sc02643b Jordan M Fletcher 1 , Katherine A Horner 2, 3 , Gail J Bartlett 1 , Guto G Rhys 1 , Andrew J Wilson 2, 3 , Derek N Woolfson 1, 4, 5
Affiliation
|
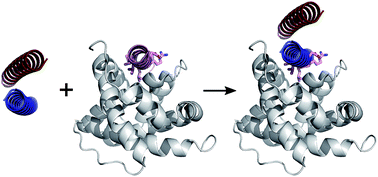
Protein-protein interactions (PPIs) play pivotal roles in the majority of biological processes. Therefore, improved approaches to target and disrupt PPIs would provide tools for chemical biology and leads for therapeutic development. PPIs with α-helical components are appealing targets given that the secondary structure is well understood and can be mimicked or stabilised to render small-molecule and constrained-peptide-based inhibitors. Here we present a strategy to target α-helix-mediated PPIs that exploits de novo coiled-coil assemblies and test this using the MCL-1/NOXA-B PPI. First, computational alanine scanning is used to identify key α-helical residues from NOXA-B that contribute to the interface. Next, these residues are grafted onto the exposed surfaces of de novo designed homodimeric or heterodimeric coiled-coil peptides. The resulting synthetic peptides selectively inhibit a cognate MCL-1/BID complex in the mid-nM range. Furthermore, the heterodimeric system affords control as inhibition occurs only when both the grafted peptide and its designed partner are present. This establishes proof of concept for exploiting peptides stabilised in de novo coiled coils as inhibitors of PPIs. This dependence on supramolecular assembly introduces new possibilities for regulation and control.
中文翻译:

从头卷曲螺旋肽作为破坏蛋白质-蛋白质相互作用的支架。
蛋白质-蛋白质相互作用 (PPI) 在大多数生物过程中发挥着关键作用。因此,靶向和破坏 PPI 的改进方法将为化学生物学提供工具并引领治疗开发。具有 α-螺旋成分的 PPI 是吸引人的目标,因为二级结构易于理解并且可以被模拟或稳定以提供小分子和基于受限肽的抑制剂。在这里,我们提出了一种针对 α-螺旋介导的 PPI 的策略,该策略利用从头卷曲线圈组件并使用 MCL-1/NOXA-B PPI 进行测试。首先,计算丙氨酸扫描用于识别来自 NOXA-B 的对界面有贡献的关键 α-螺旋残基。接下来,将这些残基移植到从头设计的同源二聚体或异源二聚体卷曲螺旋肽的暴露表面上。所得合成肽选择性地抑制中等 nM 范围内的同源 MCL-1/BID 复合物。此外,异二聚体系统提供控制,因为仅当移植肽及其设计的伙伴都存在时才会发生抑制。这确立了利用从头盘绕线圈中稳定的肽作为 PPI 抑制剂的概念证明。这种对超分子组装的依赖为调控带来了新的可能性。
更新日期:2018-08-07
中文翻译:

从头卷曲螺旋肽作为破坏蛋白质-蛋白质相互作用的支架。
蛋白质-蛋白质相互作用 (PPI) 在大多数生物过程中发挥着关键作用。因此,靶向和破坏 PPI 的改进方法将为化学生物学提供工具并引领治疗开发。具有 α-螺旋成分的 PPI 是吸引人的目标,因为二级结构易于理解并且可以被模拟或稳定以提供小分子和基于受限肽的抑制剂。在这里,我们提出了一种针对 α-螺旋介导的 PPI 的策略,该策略利用从头卷曲线圈组件并使用 MCL-1/NOXA-B PPI 进行测试。首先,计算丙氨酸扫描用于识别来自 NOXA-B 的对界面有贡献的关键 α-螺旋残基。接下来,将这些残基移植到从头设计的同源二聚体或异源二聚体卷曲螺旋肽的暴露表面上。所得合成肽选择性地抑制中等 nM 范围内的同源 MCL-1/BID 复合物。此外,异二聚体系统提供控制,因为仅当移植肽及其设计的伙伴都存在时才会发生抑制。这确立了利用从头盘绕线圈中稳定的肽作为 PPI 抑制剂的概念证明。这种对超分子组装的依赖为调控带来了新的可能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号