当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reversible Covalent Cross-Linked Polycations with Enhanced Stability and ATP-Responsive Behavior for Improved siRNA Delivery
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-08-06 00:00:00 , DOI: 10.1021/acs.biomac.8b00922 Zhanwei Zhou 1 , Minghua Zhang 1 , Yadong Liu 1 , Chenzi Li 1 , Qingyan Zhang 1 , David Oupicky 1, 2 , Minjie Sun 1
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-08-06 00:00:00 , DOI: 10.1021/acs.biomac.8b00922 Zhanwei Zhou 1 , Minghua Zhang 1 , Yadong Liu 1 , Chenzi Li 1 , Qingyan Zhang 1 , David Oupicky 1, 2 , Minjie Sun 1
Affiliation
|
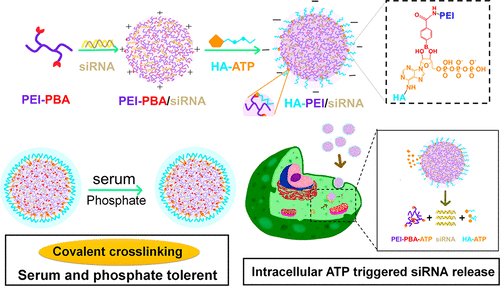
Cationic polyplex as commonly used nucleic acid carriers faced several shortcomings, such as high cytotoxicity, low serum stability, and slow cargo release at the target site. The traditional solution is covering a negative charged layer (e.g., hyaluronic acid, HA) via electrostatic interaction. However, it was far from satisfactory for the deshielding by physiological anions in circulation (e.g., serum proteins, phosphate). In this study, we proposed a new strategy of reversible covalent cross-linking to enhance stability in circulation and enable stimuli-disassembly of polyplexes in tumor cells. Here, 25k polyethylenimine (PEI) was chosen as model polycations for veriying the hypothesis. HA-PEI conjugation was formed by the cross-linking of adenosine triphosphate grafted HA (HA-ATP) with phenylboronic acid grafted PEI (PEI–PBA) via the chemical reaction between PBA and ATP. Compared with noncovalent polyplex by electrostatic interaction (HA/PEI), HA-PEI exhibited much better colloidal stability and serum stability. The covered HA-ATP layer on PEI–PBA could maintain stable in the absence of physiological anions, while HA layer on PEI in HA/PEI group showed obvious detachment after anion’s competition. More importantly, the covalent cross-linking polyplex could selectively release siRNA in the ATP rich environment of cytosol and significantly improve siRNA silence. Besides, the covalent cross-linking with HA-ATP could effectively reduce the cytotoxicity of cationic polyplex, improve the uptake by B61F10 cells and promote the endosomal escape. Consequently, this strategy of HA-PEI conjugation significantly enhanced the siRNA transfection in the absence or presence of FBS (fetal bovine serum) on B16F10 cells and CHO cells. Taken together, the reversible covalent cross-linking approach shows obvious superiority compared with the noncovalent absorption strategy. It held great potential to be developed to polish up the performance of cationic polyplex on reducing the toxicity, enhancing the serum tolerance and achieving controlled release of siRNA at target site.
中文翻译:

具有增强的稳定性和ATP响应行为的可逆共价交联聚阳离子,可改善siRNA的传递。
阳离子多聚体作为常用的核酸载体面临着许多缺点,例如高细胞毒性,低血清稳定性和在靶部位的货物释放缓慢。传统的解决方案是通过静电相互作用覆盖带负电的层(例如,透明质酸,HA)。然而,通过循环中的生理阴离子(例如血清蛋白,磷酸根)进行脱屏蔽是远远不能令人满意的。在这项研究中,我们提出了一种可逆的共价交联的新策略,以增强循环的稳定性,并使肿瘤细胞中的复合物刺激分解。在这里,选择25k聚乙烯亚胺(PEI)作为验证该假设的模型聚阳离子。HA-PEI共轭是通过三磷酸腺苷接枝的HA(HA-ATP)与苯硼酸接枝的PEI(PEI-PBA)通过PBA和ATP之间的化学反应而形成的。与通过静电相互作用的非共价复合物(HA / PEI)相比,HA-PEI具有更好的胶体稳定性和血清稳定性。在没有生理阴离子的情况下,PEI–PBA上覆盖的HA-ATP层可以保持稳定,而在HA / PEI组中PEI上的HA层在阴离子竞争后显示出明显的脱离。更重要的是,共价交联复合物可以在富含ATP的细胞溶质环境中选择性释放siRNA,并显着改善siRNA沉默。此外,与HA-ATP的共价交联可有效降低阳离子多聚体的细胞毒性,改善B61F10细胞的摄取并促进内体逃逸。因此,在B16F10细胞和CHO细胞上不存在或存在FBS(胎牛血清)的情况下,这种HA-PEI缀合策略显着增强了siRNA转染。两者合计,可逆的共价交联方法与非共价吸收策略相比显示出明显的优越性。它具有开发潜力,可增强阳离子多聚体在降低毒性,增强血清耐受性和实现siRNA在靶位点受控释放方面的性能。与非共价吸收策略相比,可逆的共价交联方法显示出明显的优越性。它具有开发潜力,可增强阳离子多聚体在降低毒性,增强血清耐受性和实现siRNA在靶位点受控释放方面的性能。与非共价吸收策略相比,可逆的共价交联方法显示出明显的优越性。它具有开发潜力,可增强阳离子多聚体在降低毒性,增强血清耐受性和实现siRNA在靶位点受控释放方面的性能。
更新日期:2018-08-06
中文翻译:

具有增强的稳定性和ATP响应行为的可逆共价交联聚阳离子,可改善siRNA的传递。
阳离子多聚体作为常用的核酸载体面临着许多缺点,例如高细胞毒性,低血清稳定性和在靶部位的货物释放缓慢。传统的解决方案是通过静电相互作用覆盖带负电的层(例如,透明质酸,HA)。然而,通过循环中的生理阴离子(例如血清蛋白,磷酸根)进行脱屏蔽是远远不能令人满意的。在这项研究中,我们提出了一种可逆的共价交联的新策略,以增强循环的稳定性,并使肿瘤细胞中的复合物刺激分解。在这里,选择25k聚乙烯亚胺(PEI)作为验证该假设的模型聚阳离子。HA-PEI共轭是通过三磷酸腺苷接枝的HA(HA-ATP)与苯硼酸接枝的PEI(PEI-PBA)通过PBA和ATP之间的化学反应而形成的。与通过静电相互作用的非共价复合物(HA / PEI)相比,HA-PEI具有更好的胶体稳定性和血清稳定性。在没有生理阴离子的情况下,PEI–PBA上覆盖的HA-ATP层可以保持稳定,而在HA / PEI组中PEI上的HA层在阴离子竞争后显示出明显的脱离。更重要的是,共价交联复合物可以在富含ATP的细胞溶质环境中选择性释放siRNA,并显着改善siRNA沉默。此外,与HA-ATP的共价交联可有效降低阳离子多聚体的细胞毒性,改善B61F10细胞的摄取并促进内体逃逸。因此,在B16F10细胞和CHO细胞上不存在或存在FBS(胎牛血清)的情况下,这种HA-PEI缀合策略显着增强了siRNA转染。两者合计,可逆的共价交联方法与非共价吸收策略相比显示出明显的优越性。它具有开发潜力,可增强阳离子多聚体在降低毒性,增强血清耐受性和实现siRNA在靶位点受控释放方面的性能。与非共价吸收策略相比,可逆的共价交联方法显示出明显的优越性。它具有开发潜力,可增强阳离子多聚体在降低毒性,增强血清耐受性和实现siRNA在靶位点受控释放方面的性能。与非共价吸收策略相比,可逆的共价交联方法显示出明显的优越性。它具有开发潜力,可增强阳离子多聚体在降低毒性,增强血清耐受性和实现siRNA在靶位点受控释放方面的性能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号