当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facile Solid-Phase Synthesis and Assessment of Nucleoside Analogs as Inhibitors of Bacterial UDP-Sugar Processing Enzymes
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-08-06 00:00:00 , DOI: 10.1021/acschembio.8b00477 Amaël G. E. Madec 1, 2 , Nathaniel S. Schocker 1, 2 , Silvano Sanchini 1, 2 , Gadam Myratgeldiyev 1, 2 , Debasis Das 1, 2 , Barbara Imperiali 1, 2
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-08-06 00:00:00 , DOI: 10.1021/acschembio.8b00477 Amaël G. E. Madec 1, 2 , Nathaniel S. Schocker 1, 2 , Silvano Sanchini 1, 2 , Gadam Myratgeldiyev 1, 2 , Debasis Das 1, 2 , Barbara Imperiali 1, 2
Affiliation
|
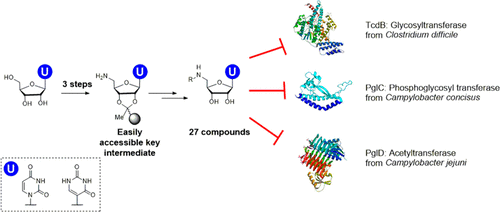
The privileged uptake of nucleosides into cells has generated interest in the development of nucleoside-analog libraries for mining new inhibitors. Of particular interest are applications in the discovery of substrate mimetic inhibitors for the growing number of identified glycan-processing enzymes in bacterial pathogens. However, the high polarity and the need for appropriate protecting group strategies for nucleosides challenges the development of synthetic approaches. Here, we report an accessible, user-friendly synthesis that branches from a common solid phase-immobilized uridinyl-amine intermediate, which can be used as a starting point for diversity-oriented synthesis. We demonstrate the generation of five series of uridinyl nucleoside analogs for investigating inhibitor structure–activity relationships. This library was screened for inhibition of representative enzymes from three functional families including a phosphoglycosyl transferase, a UDP-aminosugar acetyltransferase, and a glycosyltransferase. These candidates were taken from the Gram-negative bacteria Campylobacter concisus and Campylobacter jejuni and the Gram-positive bacterium Clostridium difficile, respectively. Inhibition studies show that specific compound series preferentially inhibit selected enzymes, with IC50 values ranging from 35 ± 7 μM to 174 ± 21 μM. Insights from the screen provide a strong foundation for further structural elaboration, to improve potency, which will be enabled by the same synthetic strategy. The solid-phase strategy was also used to synthesize pseudouridine analogs of lead compounds. Finally, the compounds were found to be nontoxic to mammalian cells, further supporting the opportunities for future development.
中文翻译:

方便的固相合成和核苷类似物作为细菌UDP-糖加工酶抑制剂的评估
核苷向细胞中的优先摄取已引起开发用于开发新抑制剂的核苷类似物库的兴趣。特别感兴趣的是在底物模拟物抑制剂的发现中的应用,所述底物模拟物抑制剂用于细菌病原体中越来越多的已确定的聚糖加工酶。然而,高极性和对核苷的适当保护基策略的需求挑战了合成方法的发展。在这里,我们报告了一种可访问的,用户友好的合成方法,该方法从固定的固相固定的尿嘧啶-胺中间体衍生而来,可以用作面向多样性的合成的起点。我们展示了五个系列的尿嘧啶核苷类似物的生成,用于研究抑制剂的结构-活性关系。筛选该文库以抑制来自三个功能家族的代表性酶,包括磷酸糖基转移酶,UDP-氨基糖乙酰基转移酶和糖基转移酶。这些候选物均来自革兰氏阴性菌空肠弯曲concisus和空肠弯曲菌和革兰氏阳性细菌艰难梭菌,分别。抑制研究表明,特定化合物系列优先抑制选定的酶,IC 50值范围从35±7μM到174±21μM。屏幕上的洞察力为进一步的结构精细化提供了坚实的基础,以提高效能,这将通过相同的合成策略来实现。固相策略还用于合成铅化合物的假尿苷类似物。最后,发现该化合物对哺乳动物细胞无毒,进一步支持了未来发展的机会。
更新日期:2018-08-06
中文翻译:

方便的固相合成和核苷类似物作为细菌UDP-糖加工酶抑制剂的评估
核苷向细胞中的优先摄取已引起开发用于开发新抑制剂的核苷类似物库的兴趣。特别感兴趣的是在底物模拟物抑制剂的发现中的应用,所述底物模拟物抑制剂用于细菌病原体中越来越多的已确定的聚糖加工酶。然而,高极性和对核苷的适当保护基策略的需求挑战了合成方法的发展。在这里,我们报告了一种可访问的,用户友好的合成方法,该方法从固定的固相固定的尿嘧啶-胺中间体衍生而来,可以用作面向多样性的合成的起点。我们展示了五个系列的尿嘧啶核苷类似物的生成,用于研究抑制剂的结构-活性关系。筛选该文库以抑制来自三个功能家族的代表性酶,包括磷酸糖基转移酶,UDP-氨基糖乙酰基转移酶和糖基转移酶。这些候选物均来自革兰氏阴性菌空肠弯曲concisus和空肠弯曲菌和革兰氏阳性细菌艰难梭菌,分别。抑制研究表明,特定化合物系列优先抑制选定的酶,IC 50值范围从35±7μM到174±21μM。屏幕上的洞察力为进一步的结构精细化提供了坚实的基础,以提高效能,这将通过相同的合成策略来实现。固相策略还用于合成铅化合物的假尿苷类似物。最后,发现该化合物对哺乳动物细胞无毒,进一步支持了未来发展的机会。



























 京公网安备 11010802027423号
京公网安备 11010802027423号