当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fucose-Functionalized Precision Glycomacromolecules Targeting Human Norovirus Capsid Protein
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-08-02 00:00:00 , DOI: 10.1021/acs.biomac.8b00829 Katharina Susanne Bücher 1 , Hao Yan 2 , Robert Creutznacher 3 , Kerstin Ruoff 4 , Alvaro Mallagaray 3 , Andrea Grafmüller 5 , Jan Sebastian Dirks 1 , Turgay Kilic 4 , Sabrina Weickert 6 , Anna Rubailo 6 , Malte Drescher 6 , Stephan Schmidt 1 , Grant Hansman 4 , Thomas Peters 3 , Charlotte Uetrecht 2, 7 , Laura Hartmann 1
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-08-02 00:00:00 , DOI: 10.1021/acs.biomac.8b00829 Katharina Susanne Bücher 1 , Hao Yan 2 , Robert Creutznacher 3 , Kerstin Ruoff 4 , Alvaro Mallagaray 3 , Andrea Grafmüller 5 , Jan Sebastian Dirks 1 , Turgay Kilic 4 , Sabrina Weickert 6 , Anna Rubailo 6 , Malte Drescher 6 , Stephan Schmidt 1 , Grant Hansman 4 , Thomas Peters 3 , Charlotte Uetrecht 2, 7 , Laura Hartmann 1
Affiliation
|
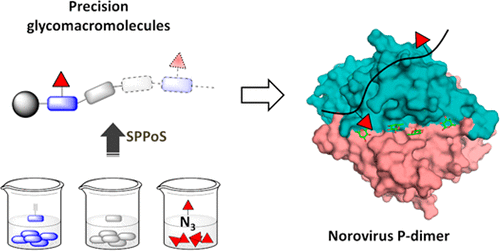
Norovirus infection is the major cause of nonbacterial gastroenteritis in humans and has been the subject of numerous studies investigating the virus’s biophysical properties and biochemical function with the aim of deriving novel and highly potent entry inhibitors to prevent infection. Recently, it has been shown that the protruding P domain dimer (P-dimer) of a GII.10 Norovirus strain exhibits two new binding sites for l-fucose in addition to the canonical binding sites. Thus, these sites provide a novel target for the design of multivalent fucose ligands as entry inhibitors of norovirus infections. In this current study, a first generation of multivalent fucose-functionalized glycomacromolecules was synthesized and applied as model structures to investigate the potential targeting of fucose binding sites in human norovirus P-dimer. Following previously established solid phase polymer synthesis, eight precision glycomacromolecules varying in number and position of fucose ligands along an oligo(amidoamine) backbone were obtained and then used in a series of binding studies applying native MS, NMR, and X-ray crystallography. We observed only one fucose per glycomacromolecule binding to one P-dimer resulting in similar binding affinities for all fucose-functionalized glycomacromolecules, which based on our current findings we attribute to the overall size of macromolecular ligands and possibly to steric hindrance.
中文翻译:

靶向人诺如病毒衣壳蛋白的功能性功能化精密糖皮质激素分子
诺如病毒感染是人类非细菌性肠胃炎的主要原因,并且已成为研究该病毒的生物物理特性和生化功能的众多研究的主题,目的在于获得新型和高效的进入抑制剂以预防感染。最近,已经表明一个GII.10诺如病毒株表现出的突出P-结构域二聚体(P-二聚体)两个新的结合位点的升-除了经典的结合位点外。因此,这些位点为设计作为诺如病毒感染的进入抑制剂的多价岩藻糖配体提供了新的靶标。在本研究中,合成了第一代多价岩藻糖功能化的糖大分子并将其用作模型结构,以研究人诺如病毒P-二聚体中岩藻糖结合位点的潜在靶向性。在先前建立的固相聚合物合成之后,获得了沿寡聚(酰胺基胺)骨架改变岩藻糖配体的数量和位置的八个精密糖大分子,然后将其用于应用天然MS,NMR和X射线晶体学的一系列结合研究中。
更新日期:2018-08-02
中文翻译:

靶向人诺如病毒衣壳蛋白的功能性功能化精密糖皮质激素分子
诺如病毒感染是人类非细菌性肠胃炎的主要原因,并且已成为研究该病毒的生物物理特性和生化功能的众多研究的主题,目的在于获得新型和高效的进入抑制剂以预防感染。最近,已经表明一个GII.10诺如病毒株表现出的突出P-结构域二聚体(P-二聚体)两个新的结合位点的升-除了经典的结合位点外。因此,这些位点为设计作为诺如病毒感染的进入抑制剂的多价岩藻糖配体提供了新的靶标。在本研究中,合成了第一代多价岩藻糖功能化的糖大分子并将其用作模型结构,以研究人诺如病毒P-二聚体中岩藻糖结合位点的潜在靶向性。在先前建立的固相聚合物合成之后,获得了沿寡聚(酰胺基胺)骨架改变岩藻糖配体的数量和位置的八个精密糖大分子,然后将其用于应用天然MS,NMR和X射线晶体学的一系列结合研究中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号