当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective fluorination of homoallylic alcohols enabled by the tuning of non-covalent interactions†
Chemical Science ( IF 8.4 ) Pub Date : 2018-08-03 00:00:00 , DOI: 10.1039/c8sc02223b Jaime A. S. Coelho 1, 2, 3, 4 , Akira Matsumoto 1, 2, 3, 4 , Manuel Orlandi 1, 4, 5, 6 , Margaret J. Hilton 1, 4, 5, 6 , Matthew S. Sigman 1, 4, 5, 6 , F. Dean Toste 1, 2, 3, 4
Chemical Science ( IF 8.4 ) Pub Date : 2018-08-03 00:00:00 , DOI: 10.1039/c8sc02223b Jaime A. S. Coelho 1, 2, 3, 4 , Akira Matsumoto 1, 2, 3, 4 , Manuel Orlandi 1, 4, 5, 6 , Margaret J. Hilton 1, 4, 5, 6 , Matthew S. Sigman 1, 4, 5, 6 , F. Dean Toste 1, 2, 3, 4
Affiliation
|
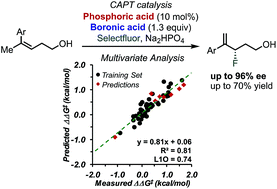
The study of the enantioselective fluorination of homoallylic alcohols via chiral anion phase transfer (CAPT) catalysis using an in situ generated directing group is described. Multivariate correlation analysis, including designer π-interaction derived parameters, revealed key structural features affecting the selectivity at the transition state (TS). Interpretation of the parameters found in the model equation highlights the key differences as well as similarities for the reaction of homoallylic and allylic substrates. A similar T-shaped π-interaction was found to occur between the substrate and the catalyst. The tuning of this crucial interaction by identification of the best combination of phosphoric acid catalyst and boronic acid directing group allowed for the development of a methodology to access γ-fluoroalkenols in typically high enantioselectivities (up to 96% ee).
中文翻译:

通过调节非共价相互作用实现对均丙醇的对映选择性氟化†
高烯丙基醇的对映选择性氟化的研究通过手性阴离子相转移(CAPT)催化使用原位描述了生成的指导组。多元相关性分析(包括设计者π交互作用得出的参数)揭示了影响过渡态(TS)选择性的关键结构特征。在模型方程式中找到的参数的解释突出显示了均烯丙基和烯丙基底物反应的关键差异和相似性。发现在底物和催化剂之间发生类似的T形π-相互作用。通过鉴定磷酸催化剂和硼酸直接基团的最佳组合来调节这种关键的相互作用,从而允许开发一种以典型的高对映选择性(至多96%ee)接近γ-氟代烯醇的方法。
更新日期:2018-08-03
中文翻译:

通过调节非共价相互作用实现对均丙醇的对映选择性氟化†
高烯丙基醇的对映选择性氟化的研究通过手性阴离子相转移(CAPT)催化使用原位描述了生成的指导组。多元相关性分析(包括设计者π交互作用得出的参数)揭示了影响过渡态(TS)选择性的关键结构特征。在模型方程式中找到的参数的解释突出显示了均烯丙基和烯丙基底物反应的关键差异和相似性。发现在底物和催化剂之间发生类似的T形π-相互作用。通过鉴定磷酸催化剂和硼酸直接基团的最佳组合来调节这种关键的相互作用,从而允许开发一种以典型的高对映选择性(至多96%ee)接近γ-氟代烯醇的方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号