Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-08-02 , DOI: 10.1016/j.cplett.2018.08.001 Jae-Hong Seo , Eugene Cha , Ho-Tae Kim
|
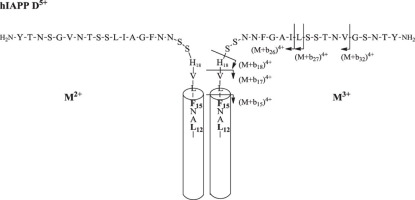
Collision-induced dissociation (CID) with ESI-MS was used to obtain structural information on early-stage aggregation of hIAPP (human islet amyloid polypeptide) monomers and oligomers. MS analysis showed that the hIAPP monomers and oligomers were multiply charged. MS/MS analysis indicated that the hIAPP monomers adopt different structures depending on the parent ion charge state. The fragmentation patterns indicated structural similarity of M2+ & M3+ and M4+ & M5+ (M = monomer). MS/MS analysis of the dimers showed that D5+ (D = dimer) comprised M2+ and M3+ subunits, and the peptide bond dissociated in the 15−37 residue region of the monomer subunit.
中文翻译:

碰撞诱导的电喷雾电离质谱解离分析人胰岛淀粉样多肽多电荷单体和二聚体
使用ESI-MS的碰撞诱导解离(CID)获得有关hIAPP(人类胰岛淀粉样多肽)单体和寡聚体早期聚集的结构信息。MS分析表明,hIAPP单体和低聚物是多电荷的。MS / MS分析表明,hIAPP单体根据母体离子电荷状态采用不同的结构。断裂模式表明M 2+和M 3+和M 4+和M 5+(M =单体)的结构相似性。对二聚体的MS / MS分析表明,D 5+(D =二聚体)包含M 2+和M 3+亚基,并且肽键在单体亚基的15-37残基区域解离。


























 京公网安备 11010802027423号
京公网安备 11010802027423号