Trends in Food Science & Technology ( IF 15.3 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.tifs.2018.07.026 Shahzeb Khan , Muhammad Imran , Tariq Tahir Butt , Syed Wadood Ali Shah , Muhammad Sohail , Arif Malik , Srijit Das , Hnin Ei Thu , Aishah Adam , Zahid Hussain
|
Background
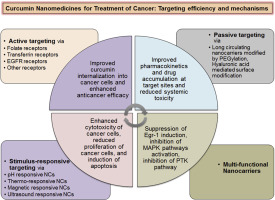
Cancer is a group of diseases involving an abnormal growth of cells which tend to proliferate in an uncontrolled fashion and in some cases metastasize to the surrounding tissues (malignancy). Resistance to chemotherapy is typically intrinsic (heterogeneity); however, acquired resistance has also become prevelant due to multiple factors including expression of energy-dependent transporters causing expulsion of internalized drug contents extracellular, insensitivity of tumor cells to drug-induced apoptosis, and induction of drug-detoxifying mechanisms. Curcumin (CUR) has gained widespread recognition due to remarkable anticancer, anti-mutagenic, and anti-metastasizing potentials via downregulation of proliferation of cancer cells and induction of apoptosis. Nevertheless, pharmaceutical significance and therapeutic feasibility of CUR is restricted due to intrinsic physicochemical characteristics including poor aqueous solubility, inadequate biological stability, low bioavailability, and short half-life.
Scope and approach
Owing to these pharmaceutical limitations of CUR, nanodelivery systems have attained remarkable fascination in the recent years. Therefore, this review was aimed to overview and critically ponders recent developments in improving anticancer viability of CUR.
Key findings and conclusion
Critical analysis of the literature revealed that nanodelivery systems showed promising efficiency in achieving tumor specific targetability, maximizing internalization of drugs into cancer cells, mitigating tumor metastasis, as well as improving anticancer efficacy of CUR. Moreover, nanocarrier-mediated improved pharmacokinetics, drug accumulation, induced promising cytotoxicity, and enhanced anticancer efficacy by suppressing Egr-1 induction, Mitogen-activated protein kinase (MAPK) pathway, and protein tyrosine kinase (PTK) cascades while mitigating the progression of tumor, have also been discussed.
中文翻译:

姜黄素基纳米药物作为治疗癌症的有效纳米平台:逆转癌症耐药性,快速内在化和提高的抗癌功效方面的新进展
背景
癌症是一组涉及细胞异常生长的疾病,这些细胞倾向于以不受控制的方式增殖,并在某些情况下转移到周围组织(恶性肿瘤)。对化学疗法的抵抗力通常是内在的(异质性)。然而,由于多种因素,包括获得能量依赖性转运蛋白的表达引起细胞内在化的内含物排出细胞外,肿瘤细胞对药物诱导的凋亡不敏感以及药物解毒机制的诱导,获得性耐药也变得很流行。姜黄素(CUR)得到了广泛的认可,由于显着的抗癌,抗突变,防转移性潜力通过下调癌细胞的增殖并诱导凋亡。然而,由于固有的物理化学特征包括不良的水溶性,不足的生物稳定性,低的生物利用度和短的半衰期,CUR的药学意义和治疗可行性受到限制。
范围和方法
由于CUR的这些药物局限性,近年来,纳米递送系统获得了极大的吸引力。因此,本综述旨在概述并批判性地研究改善CUR的抗癌活性的最新进展。
主要发现和结论
对文献的批判性分析显示,纳米递送系统在实现肿瘤特异性靶向性,最大程度地将药物内化到癌细胞,减轻肿瘤转移以及提高CUR的抗癌效力方面显示出令人鼓舞的效率。此外,通过抑制Egr-1诱导,丝裂原激活的蛋白激酶(MAPK)途径和蛋白酪氨酸激酶(PTK)级联,纳米载体介导的改善的药代动力学,药物蓄积,诱导有希望的细胞毒性和增强的抗癌功效,同时减轻了肿瘤的进展,也已经讨论过。


























 京公网安备 11010802027423号
京公网安备 11010802027423号