当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The coordination chemistry of CmIII, AmIII, and AcIII in nitrate solutions: an actinide L3-edge EXAFS study†‡
Chemical Science ( IF 8.4 ) Pub Date : 2018-08-01 00:00:00 , DOI: 10.1039/c8sc02270d Maryline G Ferrier 1 , Benjamin W Stein 1 , Sharon E Bone 1 , Samantha K Cary 1 , Alexander S Ditter 1, 2 , Stosh A Kozimor 1 , Juan S Lezama Pacheco 3 , Veronika Mocko 1 , Gerald T Seidler 2
Chemical Science ( IF 8.4 ) Pub Date : 2018-08-01 00:00:00 , DOI: 10.1039/c8sc02270d Maryline G Ferrier 1 , Benjamin W Stein 1 , Sharon E Bone 1 , Samantha K Cary 1 , Alexander S Ditter 1, 2 , Stosh A Kozimor 1 , Juan S Lezama Pacheco 3 , Veronika Mocko 1 , Gerald T Seidler 2
Affiliation
|
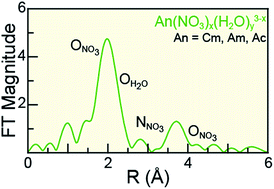
Understanding actinide(III) (AnIII = CmIII, AmIII, AcIII) solution-phase speciation is critical for controlling many actinide processing schemes, ranging from medical applications to reprocessing of spent nuclear fuel. Unfortunately, in comparison to most elements in the periodic table, AnIII speciation is often poorly defined in complexing aqueous solutions and in organic media. This neglect – in large part – is a direct result of the radioactive properties of these elements, which make them difficult to handle and acquire. Herein, we surmounted some of the handling challenges associated with these exotic 5f-elements and characterized CmIII, AmIII, and AcIII using AnIII L3-edge X-ray absorption spectroscopy (XAS) as a function of increasing nitric acid (HNO3) concentration. Our results revealed that actinide aquo ions, An(H2O)x3+ (x = 9.6 ± 0.7, 8.9 ± 0.8, and 10.0 ± 0.9 for CmIII, AmIII, and AcIII), were the dominant species in dilute HNO3 (0.05 M). In concentrated HNO3 (16 M), shell-by-shell fitting of the extended X-ray fine structure (EXAFS) data showed the nitrate complexation increased, such that the average stoichiometries of Cm(NO3)4.1±0.7(H2O)5.7±1.3(1.1±0.2)−, Am(NO3)3.4±0.7(H2O)5.4±0.5(0.4±0.1)−, and Ac(NO3)2.3±1.7(H2O)8.3±5.2(0.7±0.5)+ were observed. Data obtained at the intermediate HNO3 concentration (4 M) were modeled as a linear combination of the 0.05 and 16 M spectra. For all three metals, the intermediate models showed larger contributions from the 0.05 M HNO3 spectra than from the 16 M HNO3 spectra. Additionally, these efforts enabled the Cm–NO3 and Ac–NO3 distances to be measured for the first time. Moreover, the AnIII L3-edge EXAFS results, contribute to the growing body of knowledge associated with CmIII, AmIII, and AcIII coordination chemistry, in particular toward advancing understanding of AnIII solution phase speciation.
中文翻译:

硝酸盐溶液中 CmIII、AmIII 和 AcIII 的配位化学:锕系元素 L3 边缘 EXAFS 研究†‡
了解锕系元素( III )(An III = Cm III、Am III、Ac III)溶液相形态对于控制许多锕系元素处理方案(从医疗应用到乏核燃料后处理)至关重要。不幸的是,与周期表中的大多数元素相比,络合水溶液和有机介质中的III形态通常很难确定。这种忽视在很大程度上是这些元素的放射性特性的直接结果,这使得它们难以处理和获取。在此,我们克服了与这些奇异 5f 元素相关的一些处理挑战,并使用 An III L 3边 X 射线吸收光谱 (XAS) 表征了 Cm III、Am III和 Ac III随硝酸浓度增加的函数 ( HNO 3 ) 浓度。我们的结果表明,锕系元素水合离子 An(H 2 O) x 3+(对于 Cm III、Am III和 Ac III , x = 9.6 ± 0.7、8.9 ± 0.8 和 10.0 ± 0.9 )是稀溶液中的主要物种。 HNO 3 (0.05 M)。在浓HNO 3 (16 M)中,扩展X射线精细结构(EXAFS)数据的逐壳拟合显示硝酸盐络合作用增加,使得Cm(NO 3 ) 4.1 ± 0.7 ( H 2 O) 5.7±1.3 (1.1±0.2)−、Am(NO 3 ) 3.4±0.7 (H 2 O) 5.4±0.5 (0.4±0.1)−、Ac(NO 3 ) 2.3±1.7 (H 2 O) 8.3观察到±5.2 (0.7±0.5)+ 。在中间 HNO 3浓度 (4 M) 下获得的数据被建模为 0.05 和 16 M 光谱的线性组合。对于所有三种金属,中间模型显示 0.05 M HNO 3光谱的贡献大于 16 M HNO 3光谱的贡献。此外,这些努力使得 Cm-NO 3和 Ac-NO 3距离首次被测量。此外,An III L 3边 EXAFS 结果有助于不断增长与 Cm III、Am III和 Ac III相关的知识体系配位化学,特别是促进对III溶液相形态的理解。
更新日期:2018-08-01
中文翻译:

硝酸盐溶液中 CmIII、AmIII 和 AcIII 的配位化学:锕系元素 L3 边缘 EXAFS 研究†‡
了解锕系元素( III )(An III = Cm III、Am III、Ac III)溶液相形态对于控制许多锕系元素处理方案(从医疗应用到乏核燃料后处理)至关重要。不幸的是,与周期表中的大多数元素相比,络合水溶液和有机介质中的III形态通常很难确定。这种忽视在很大程度上是这些元素的放射性特性的直接结果,这使得它们难以处理和获取。在此,我们克服了与这些奇异 5f 元素相关的一些处理挑战,并使用 An III L 3边 X 射线吸收光谱 (XAS) 表征了 Cm III、Am III和 Ac III随硝酸浓度增加的函数 ( HNO 3 ) 浓度。我们的结果表明,锕系元素水合离子 An(H 2 O) x 3+(对于 Cm III、Am III和 Ac III , x = 9.6 ± 0.7、8.9 ± 0.8 和 10.0 ± 0.9 )是稀溶液中的主要物种。 HNO 3 (0.05 M)。在浓HNO 3 (16 M)中,扩展X射线精细结构(EXAFS)数据的逐壳拟合显示硝酸盐络合作用增加,使得Cm(NO 3 ) 4.1 ± 0.7 ( H 2 O) 5.7±1.3 (1.1±0.2)−、Am(NO 3 ) 3.4±0.7 (H 2 O) 5.4±0.5 (0.4±0.1)−、Ac(NO 3 ) 2.3±1.7 (H 2 O) 8.3观察到±5.2 (0.7±0.5)+ 。在中间 HNO 3浓度 (4 M) 下获得的数据被建模为 0.05 和 16 M 光谱的线性组合。对于所有三种金属,中间模型显示 0.05 M HNO 3光谱的贡献大于 16 M HNO 3光谱的贡献。此外,这些努力使得 Cm-NO 3和 Ac-NO 3距离首次被测量。此外,An III L 3边 EXAFS 结果有助于不断增长与 Cm III、Am III和 Ac III相关的知识体系配位化学,特别是促进对III溶液相形态的理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号