当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Soluble Zwitterionic Poly(sulfobetaine) Destabilizes Proteins
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-07-31 00:00:00 , DOI: 10.1021/acs.biomac.8b01120 Lydia Kisley , Kali A. Serrano , Caitlin M. Davis , Drishti Guin , Elizabeth A. Murphy , Martin Gruebele , Deborah E. Leckband
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-07-31 00:00:00 , DOI: 10.1021/acs.biomac.8b01120 Lydia Kisley , Kali A. Serrano , Caitlin M. Davis , Drishti Guin , Elizabeth A. Murphy , Martin Gruebele , Deborah E. Leckband
|
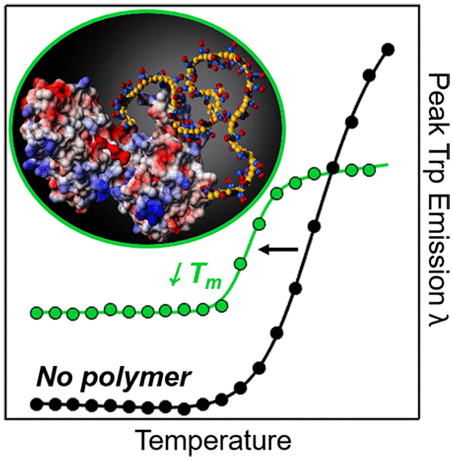
The widespread interest in neutral, water-soluble polymers such as poly(ethylene glycol) (PEG) and poly(zwitterions) such as poly(sulfobetaine) (pSB) for biomedical applications is due to their widely assumed low protein binding. Here we demonstrate that pSB chains in solution can interact with proteins directly. Moreover, pSB can reduce the thermal stability and increase the protein folding cooperativity relative to proteins in buffer or in PEG solutions. Polymer-dependent changes in the tryptophan fluorescence spectra of three structurally-distinct proteins reveal that soluble, 100 kDa pSB interacts directly with all three proteins and changes both the local polarity near tryptophan residues and the protein conformation. Thermal denaturation studies show that the protein melting temperatures decrease by as much as ∼1.9 °C per weight percent of polymer and that protein folding cooperativity increases by as much as ∼130 J mol–1 K–1 per weight percent of polymer. The exact extent of the changes is protein-dependent, as some proteins exhibit increased stability, whereas others experience decreased stability at high soluble pSB concentrations. These results suggest that pSB is not universally protein-repellent and that its efficacy in biotechnological applications will depend on the specific proteins used.
中文翻译:

可溶性两性离子聚(磺基甜菜碱)使蛋白质不稳定
对于生物医学应用,中性水溶性聚合物(例如聚乙二醇)(PEG)和聚两性离子(例如聚磺基甜菜碱)(pSB)引起了广泛关注,这是由于它们广泛的低蛋白结合性。在这里,我们证明溶液中的pSB链可以直接与蛋白质相互作用。此外,相对于缓冲液或PEG溶液中的蛋白质,pSB可以降低热稳定性并增加蛋白质折叠的协同作用。三种结构不同的蛋白质在色氨酸荧光光谱中的聚合物依赖性变化表明,可溶的100 kDa pSB与所有这三种蛋白质直接相互作用,并改变了色氨酸残基附近的局部极性和蛋白质构象。热变性研究表明,蛋白质的解链温度降低了约1倍。–1 K –1(以聚合物的重量百分比计)。变化的确切程度是蛋白质依赖性的,因为某些蛋白质表现出增加的稳定性,而另一些蛋白质在高可溶性pSB浓度下则表现出下降的稳定性。这些结果表明,pSB不能普遍排斥蛋白质,其在生物技术应用中的功效将取决于所用的特定蛋白质。
更新日期:2018-07-31
中文翻译:

可溶性两性离子聚(磺基甜菜碱)使蛋白质不稳定
对于生物医学应用,中性水溶性聚合物(例如聚乙二醇)(PEG)和聚两性离子(例如聚磺基甜菜碱)(pSB)引起了广泛关注,这是由于它们广泛的低蛋白结合性。在这里,我们证明溶液中的pSB链可以直接与蛋白质相互作用。此外,相对于缓冲液或PEG溶液中的蛋白质,pSB可以降低热稳定性并增加蛋白质折叠的协同作用。三种结构不同的蛋白质在色氨酸荧光光谱中的聚合物依赖性变化表明,可溶的100 kDa pSB与所有这三种蛋白质直接相互作用,并改变了色氨酸残基附近的局部极性和蛋白质构象。热变性研究表明,蛋白质的解链温度降低了约1倍。–1 K –1(以聚合物的重量百分比计)。变化的确切程度是蛋白质依赖性的,因为某些蛋白质表现出增加的稳定性,而另一些蛋白质在高可溶性pSB浓度下则表现出下降的稳定性。这些结果表明,pSB不能普遍排斥蛋白质,其在生物技术应用中的功效将取决于所用的特定蛋白质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号