Journal of the American Society for Mass Spectrometry ( IF 3.2 ) Pub Date : 2018-07-31 , DOI: 10.1007/s13361-018-2015-x Martine Beaufour 1 , David Ginguené 1 , Rémy Le Meur 1, 2, 3 , Bertrand Castaing 1 , Martine Cadene 1
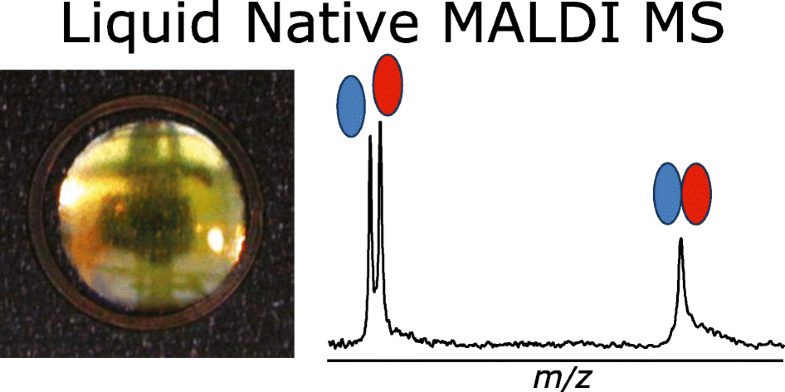
Native mass spectrometry (MS) encompasses methods to keep noncovalent interactions of biomolecular complexes intact in the gas phase throughout the instrument and to measure the mass-to-charge ratios of supramolecular complexes directly in the mass spectrometer. Electrospray ionization (ESI) in nondenaturing conditions is now an established method to characterize noncovalent systems. Matrix-assisted laser desorption/ionization (MALDI), on the other hand, consumes low quantities of samples and largely tolerates contaminants, making it a priori attractive for native MS. However, so-called native MALDI approaches have so far been based on solid deposits, where the rapid transition of the sample through a solid state can engender the loss of native conformations. Here we present a new method for native MS based on liquid deposits and MALDI ionization, unambiguously detecting intact noncovalent protein complexes by direct desorption from a liquid spot for the first time. To control for aggregation, we worked with HUαβ, a heterodimer that does not spontaneously rearrange into homodimers in solution. Screening through numerous matrix solutions to observe first the monomeric protein, then the dimer complex, we settled on a nondenaturing binary matrix solution composed of acidic and basic organic matrices in glycerol, which is stable in vacuo. The role of temporal and spatial laser irradiation patterns was found to be critical. Both a protein-protein and a protein-ligand complex could be observed free of aggregation. To minimize gas-phase dissociation, source parameters were optimized to achieve a conservation of complexes above 50% for both systems.
ᅟ
中文翻译:

液相天然MALDI质谱检测蛋白质-蛋白质复合物
天然质谱(MS)涵盖了在整个仪器中保持气相中生物分子复合物的非共价相互作用完整并直接在质谱仪中测量超分子复合物的质荷比的方法。非变性条件下的电喷雾电离(ESI)现在已成为表征非共价体系的既定方法。另一方面,基质辅助激光解吸/电离(MALDI)消耗的样品量少,并且对污染物的耐受性强,因此具有先验性对本机MS具有吸引力。然而,迄今为止,所谓的天然MALDI方法已经基于固体沉积物,其中样品通过固态的快速转变会导致天然构象的损失。在这里,我们提出了一种基于液体沉积物和MALDI电离的天然MS的新方法,这是首次通过直接从液体斑点中解吸来明确检测完整的非共价蛋白复合物。为了控制聚集,我们使用了HUαβ,这是一种异二聚体,它不会自发地重排成溶液中的同二聚体。通过众多基质溶液进行筛选,首先观察单体蛋白,然后观察二聚体复合物,然后我们在由甘油中的酸性和碱性有机基质组成的非变性二元基质溶液中进行沉淀,该溶液在真空中稳定。发现时间和空间激光照射模式的作用至关重要。可以观察到蛋白质-蛋白质和蛋白质-配体复合物都没有聚集。为了最大程度地减少气相离解,对两个系统的源参数进行了优化,以使络合物的守恒度保持在50%以上。

ᅟ


























 京公网安备 11010802027423号
京公网安备 11010802027423号