当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Engineering an Automaturing Transglutaminase with Enhanced Thermostability by Genetic Code Expansion with Two Codon Reassignments
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-07-31 00:00:00 , DOI: 10.1021/acssynbio.8b00157 Kazumasa Ohtake , Takahito Mukai , Fumie Iraha , Mihoko Takahashi , Ken-ichi Haruna 1 , Masayo Date 1 , Keiichi Yokoyama 1 , Kensaku Sakamoto
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-07-31 00:00:00 , DOI: 10.1021/acssynbio.8b00157 Kazumasa Ohtake , Takahito Mukai , Fumie Iraha , Mihoko Takahashi , Ken-ichi Haruna 1 , Masayo Date 1 , Keiichi Yokoyama 1 , Kensaku Sakamoto
Affiliation
|
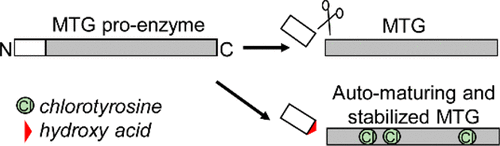
In the present study, we simultaneously incorporated two types of synthetic components into microbial transglutaminase (MTG) from Streptoverticillium mobaraense to enhance the utility of this industrial enzyme. The first amino acid, 3-chloro-l-tyrosine, was incorporated into MTG in response to in-frame UAG codons to substitute for the 15 tyrosine residues separately. The two substitutions at positions 20 and 62 were found to each increase thermostability of the enzyme, while the seven substitutions at positions 24, 34, 75, 146, 171, 217, and 310 exhibited neutral effects. Then, these two stabilizing chlorinations were combined with one of the neutral ones, and the most stabilized variant was found to contain 3-chlorotyrosines at positions 20, 62, and 171, exhibiting a half-life 5.1-fold longer than that of the wild-type enzyme at 60 °C. Next, this MTG variant was further modified by incorporating the α-hydroxy acid analogue of Nε-allyloxycarbonyl-l-lysine (AlocKOH), specified by the AGG codon, at the end of the N-terminal inhibitory peptide. We used an Escherichia coli strain previously engineered to have a synthetic genetic code with two codon reassignments for synthesizing MTG variants containing both 3-chlorotyrosine and AlocKOH. The ester bond, thus incorporated into the main chain, efficiently self-cleaved under alkaline conditions (pH 11.0), achieving the autonomous maturation of the thermostabilized MTG. The results suggested that synthetic genetic codes with multiple codon reassignments would be useful for developing the novel designs of enzymes.
中文翻译:

通过带有两个密码子重分配的遗传密码扩展工程设计具有增强的热稳定性的自动转谷氨酰胺酶
在本研究中,我们同时将两种合成成分掺入了来自莫原链霉菌的微生物转谷氨酰胺酶(MTG)中,以增强该工业酶的实用性。第一个氨基酸,3-氯-升-酪氨酸,响应于框内的UAG密码子被掺入MTG中,以分别替代15个酪氨酸残基。发现在位置20和62处的两个取代各自增加了酶的热稳定性,而在位置24、34、75、146、171、217和310处的七个取代表现出中性作用。然后,将这两种稳定的氯化反应与一种中性氯化反应结合使用,发现最稳定的变体在20、62和171位含有3-氯酪氨酸,半衰期比野生半衰期长5.1倍。型酶在60°C下。接着,将该MTG变体进一步通过结合的α羟基酸类似物改性Ñ ε -allyloxycarbonyl-升-赖氨酸(AlocKOH),由AGG密码子指定,位于N端抑制肽的末端。我们使用了以前经过改造的大肠杆菌菌株,该菌株经过人工合成,具有两个密码子重分配的合成遗传密码,用于合成同时含有3-氯酪氨酸和AlocKOH的MTG变体。因此,结合到主链中的酯键在碱性条件(pH 11.0)下有效地自我裂解,实现了热稳定MTG的自主成熟。结果表明具有多个密码子重新分配的合成遗传密码将有助于开发新颖的酶设计。
更新日期:2018-07-31
中文翻译:

通过带有两个密码子重分配的遗传密码扩展工程设计具有增强的热稳定性的自动转谷氨酰胺酶
在本研究中,我们同时将两种合成成分掺入了来自莫原链霉菌的微生物转谷氨酰胺酶(MTG)中,以增强该工业酶的实用性。第一个氨基酸,3-氯-升-酪氨酸,响应于框内的UAG密码子被掺入MTG中,以分别替代15个酪氨酸残基。发现在位置20和62处的两个取代各自增加了酶的热稳定性,而在位置24、34、75、146、171、217和310处的七个取代表现出中性作用。然后,将这两种稳定的氯化反应与一种中性氯化反应结合使用,发现最稳定的变体在20、62和171位含有3-氯酪氨酸,半衰期比野生半衰期长5.1倍。型酶在60°C下。接着,将该MTG变体进一步通过结合的α羟基酸类似物改性Ñ ε -allyloxycarbonyl-升-赖氨酸(AlocKOH),由AGG密码子指定,位于N端抑制肽的末端。我们使用了以前经过改造的大肠杆菌菌株,该菌株经过人工合成,具有两个密码子重分配的合成遗传密码,用于合成同时含有3-氯酪氨酸和AlocKOH的MTG变体。因此,结合到主链中的酯键在碱性条件(pH 11.0)下有效地自我裂解,实现了热稳定MTG的自主成熟。结果表明具有多个密码子重新分配的合成遗传密码将有助于开发新颖的酶设计。


























 京公网安备 11010802027423号
京公网安备 11010802027423号