当前位置:
X-MOL 学术
›
J. Anal. Appl. Pyrol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Surface Chemistry of Electronic Cigarette Electrical Heating Coils: Effects of Metal Type on Propylene Glycol Thermal Decomposition
Journal of Analytical and Applied Pyrolysis ( IF 6 ) Pub Date : 2018-09-01 , DOI: 10.1016/j.jaap.2018.07.019 Najat A Saliba 1, 2 , Ahmad El Hellani 1, 2 , Edward Honein 3 , Rola Salman 2, 4 , Soha Talih 2, 4 , Joseph Zeaiter 3 , Alan Shihadeh 2, 4
Journal of Analytical and Applied Pyrolysis ( IF 6 ) Pub Date : 2018-09-01 , DOI: 10.1016/j.jaap.2018.07.019 Najat A Saliba 1, 2 , Ahmad El Hellani 1, 2 , Edward Honein 3 , Rola Salman 2, 4 , Soha Talih 2, 4 , Joseph Zeaiter 3 , Alan Shihadeh 2, 4
Affiliation
|
Introduction
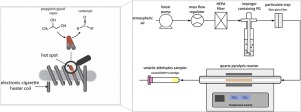
Carbonyls, a class of compounds strongly linked to pulmonary disease in smokers, are probably the most reported non-nicotine toxicants found aerosols. Reported emissions vary from negligible quantities to those far exceeding combustible cigarettes. Observations of high emissions are commonly attributed to "dry puffing", whereby the ECIG heating filament runs dry of liquid and reaches temperatures that induce thermal degradation of the ECIG vapor components at the filament's metal surface. Using a pyrolysis flow reactor, in this study we examined the potential role of surface chemistry in the formation of carbonyl compounds in ECIGs, and whether the different commercially available filament materials could potentially impact their toxicant emissions through catalysis. This information could inform nascent efforts to regulate the design of ECIGs for public health ends. Methods
Nitrogen or air saturated with propylene glycol vapor was drawn through a temperature and residence time controlled tubular quartz pyrolysis flow reactor in which nichrome, Kanthal, or stainless steel ECIG heating filament wires were inserted. A control condition with no inserted wire was also included. Concentrations of carbonyl products at the reactor outlet were measured as a function of temperature, heating filament wire material, and carrier gas composition (N2 vs air). Carbonyls were sampled using DNPH cartridges and analyzed by HPLC. Results
ECIG heating filament wires were found to have a strong catalytic effect. Carbonyl formation initiated at temperatures lower than 250°C in the presence of the metallic wires, compared to 460°C without them. Carbonyl formation was found to be a function of the material of construction, and whether the wire was new or aged. New nichrome wires were the least reactive, but when aged they exhibited the highest reactivity. Carbonyls were formed via dehydration or oxidation reactions of PG. Conclusions
Carbonyl formation chemistry is catalyzed by commonly used ECIG heating filament materials, at temperatures that are well below those expected during "dry puffing". The variability in the distribution and yield of carbonyl compounds across ECIG filament materials suggests that this heretofore unaccounted variable may partially explain the wide ranges reported in the literature to date. More importantly, it suggests that ECIG construction materials may be an important variable for regulations designed to protect public health.
中文翻译:

电子烟电加热线圈的表面化学:金属类型对丙二醇热分解的影响
简介 羰基化合物是一类与吸烟者肺部疾病密切相关的化合物,可能是在气溶胶中发现的最常见的非尼古丁有毒物质。报告的排放量从微不足道的数量到远远超过可燃卷烟的排放量不等。对高排放的观察通常归因于“干膨化”,即 ECIG 加热灯丝在没有液体的情况下运行并达到导致灯丝金属表面的 ECIG 蒸汽成分热降解的温度。在这项研究中,我们使用热解流动反应器检查了表面化学在 ECIG 中羰基化合物形成中的潜在作用,以及不同的市售长丝材料是否可能通过催化影响其有毒物质的排放。这些信息可以为新的努力提供信息,以规范 ECIG 的设计以达到公共卫生目的。方法 将饱和丙二醇蒸气的氮气或空气通过温度和停留时间控制的管状石英热解流动反应器抽取,其中插入镍铬合金、Kanthal 或不锈钢 ECIG 加热丝线。还包括没有插入电线的控制条件。测量反应器出口处的羰基产物浓度作为温度、加热丝线材料和载气组成(N2 与空气)的函数。使用 DNPH 小柱对羰基化合物进行采样并通过 HPLC 进行分析。结果发现ECIG加热丝线具有很强的催化作用。在金属丝存在的情况下,在低于 250°C 的温度下开始形成羰基,与没有它们的 460°C 相比。发现羰基的形成是结构材料的函数,以及导线是新的还是老化的。新的镍铬合金线的反应性最低,但老化时它们表现出最高的反应性。羰基化合物是通过 PG 的脱水或氧化反应形成的。结论 羰基形成化学由常用的 ECIG 加热丝材料催化,其温度远低于“干膨化”期间的预期温度。ECIG 灯丝材料中羰基化合物的分布和产量的可变性表明,这个迄今为止未解释的变量可能部分解释了迄今为止文献中报道的广泛范围。更重要的是,
更新日期:2018-09-01
中文翻译:

电子烟电加热线圈的表面化学:金属类型对丙二醇热分解的影响
简介 羰基化合物是一类与吸烟者肺部疾病密切相关的化合物,可能是在气溶胶中发现的最常见的非尼古丁有毒物质。报告的排放量从微不足道的数量到远远超过可燃卷烟的排放量不等。对高排放的观察通常归因于“干膨化”,即 ECIG 加热灯丝在没有液体的情况下运行并达到导致灯丝金属表面的 ECIG 蒸汽成分热降解的温度。在这项研究中,我们使用热解流动反应器检查了表面化学在 ECIG 中羰基化合物形成中的潜在作用,以及不同的市售长丝材料是否可能通过催化影响其有毒物质的排放。这些信息可以为新的努力提供信息,以规范 ECIG 的设计以达到公共卫生目的。方法 将饱和丙二醇蒸气的氮气或空气通过温度和停留时间控制的管状石英热解流动反应器抽取,其中插入镍铬合金、Kanthal 或不锈钢 ECIG 加热丝线。还包括没有插入电线的控制条件。测量反应器出口处的羰基产物浓度作为温度、加热丝线材料和载气组成(N2 与空气)的函数。使用 DNPH 小柱对羰基化合物进行采样并通过 HPLC 进行分析。结果发现ECIG加热丝线具有很强的催化作用。在金属丝存在的情况下,在低于 250°C 的温度下开始形成羰基,与没有它们的 460°C 相比。发现羰基的形成是结构材料的函数,以及导线是新的还是老化的。新的镍铬合金线的反应性最低,但老化时它们表现出最高的反应性。羰基化合物是通过 PG 的脱水或氧化反应形成的。结论 羰基形成化学由常用的 ECIG 加热丝材料催化,其温度远低于“干膨化”期间的预期温度。ECIG 灯丝材料中羰基化合物的分布和产量的可变性表明,这个迄今为止未解释的变量可能部分解释了迄今为止文献中报道的广泛范围。更重要的是,



























 京公网安备 11010802027423号
京公网安备 11010802027423号