当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A dual response fluorescent sensor for HNO and S2− ions using a Cu(ii) complex based probe assisted by detailed DFT studies†
Dalton Transactions ( IF 4 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1039/c8dt02784f Ananya Dutta 1, 2, 3 , Rabiul Alam 1, 2, 3 , Abu Saleh Musha Islam 1, 2, 3 , Arpan Dutta 1, 2, 3 , Mahammad Ali 1, 2, 3, 4, 5
Dalton Transactions ( IF 4 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1039/c8dt02784f Ananya Dutta 1, 2, 3 , Rabiul Alam 1, 2, 3 , Abu Saleh Musha Islam 1, 2, 3 , Arpan Dutta 1, 2, 3 , Mahammad Ali 1, 2, 3, 4, 5
Affiliation
|
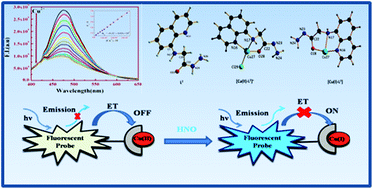
A Cu(II) based sensor (1) prepared by the complexation between (quinolin-8-ylamino)-acetic acid hydrazide (L2) and Cu2+ ions has been developed for a highly sensitive and selective recognition of HNO and S2− over other biologically abundant anions with prominent enhancement in absorption and emission intensities. The sensor (1) shows weak fluorescence due to ET (electron transfer) but upon addition of HNO and S2− a large enhancement in the fluorescence intensity (F.I.) was observed over other possible competitive anions on the basis of reduction of Cu(II) to Cu(I) and formation of CuS, respectively. The 1 : 1 complexation was characterized by mass spectrometry (MS), elemental analysis and Job's plot. The corresponding Kf value was evaluated to be (4.934 ± 0.05) × 104 M−1 for Cu2+ from UV-Vis absorption titration. Quantum yields of L2 and [Cu-L2 + S2−] and [Cu-L2 + HNO] complexes in acetonitrile (CH3CN) are found to be 0.107, 0.09 and 0.07, respectively, using quinine sulphate as the standard.
中文翻译:

使用基于Cu(ii)配合物的探针 对HNO和S 2-离子的双响应荧光传感器,并辅以详细的DFT研究†
通过(喹啉-8-基氨基)-乙酸酰肼(L 2)和Cu 2+离子的络合制备的基于Cu(II)的传感器(1)已被开发用于高度敏感和选择性地识别HNO和S 2 −超过其他生物丰富的阴离子,吸收和发射强度显着提高。传感器(1)由于ET(电子转移)而显示出弱荧光,但是在添加HNO和S 2-的情况下,由于铜(II)的还原,与其他可能的竞争性阴离子相比,荧光强度(FI)大大提高。)到Cu(I)和CuS的形成。1:1络合物通过质谱(MS),元素分析和Job's图表征。通过UV-Vis吸收滴定,对于Cu 2+,相应的K f值被评估为(4.934±0.05)×10 4 M -1。使用硫酸奎宁作为溶剂,发现乙腈(CH 3 CN)中L 2和[ Cu-L 2 + S 2− ]和[ Cu-L 2 + HNO]配合物的量子产率分别为0.107、0.09和0.07。标准。
更新日期:2018-07-25
中文翻译:

使用基于Cu(ii)配合物的探针 对HNO和S 2-离子的双响应荧光传感器,并辅以详细的DFT研究†
通过(喹啉-8-基氨基)-乙酸酰肼(L 2)和Cu 2+离子的络合制备的基于Cu(II)的传感器(1)已被开发用于高度敏感和选择性地识别HNO和S 2 −超过其他生物丰富的阴离子,吸收和发射强度显着提高。传感器(1)由于ET(电子转移)而显示出弱荧光,但是在添加HNO和S 2-的情况下,由于铜(II)的还原,与其他可能的竞争性阴离子相比,荧光强度(FI)大大提高。)到Cu(I)和CuS的形成。1:1络合物通过质谱(MS),元素分析和Job's图表征。通过UV-Vis吸收滴定,对于Cu 2+,相应的K f值被评估为(4.934±0.05)×10 4 M -1。使用硫酸奎宁作为溶剂,发现乙腈(CH 3 CN)中L 2和[ Cu-L 2 + S 2− ]和[ Cu-L 2 + HNO]配合物的量子产率分别为0.107、0.09和0.07。标准。



























 京公网安备 11010802027423号
京公网安备 11010802027423号