当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Non‐Covalent Substrate Directed Enantioselective Heck Desymmetrization of cis‐Cyclohex‐4‐ene‐1,2‐diol: Synthesis of all cis Chiral 5‐Aryl‐cyclohex‐3‐ene‐1,2‐diols and Mechanistic Investigation
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-08-14 , DOI: 10.1002/adsc.201800785 Ricardo A. Angnes 1, 2 , Lee M. Thompson 1 , Mark S. Mashuta 1 , Carlos R. D. Correia 2 , Gerald B. Hammond 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-08-14 , DOI: 10.1002/adsc.201800785 Ricardo A. Angnes 1, 2 , Lee M. Thompson 1 , Mark S. Mashuta 1 , Carlos R. D. Correia 2 , Gerald B. Hammond 1
Affiliation
|
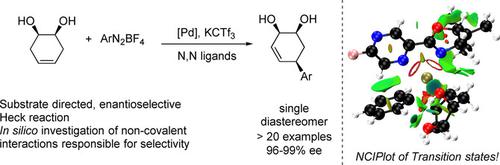
Enantioselective substrate directed Heck reactions are desirable for the stereoselective synthesis of complex molecules. However, due to the coordination requirements of both chiral ligands and directing groups, such methodologies are underdeveloped. We report herein the desymmetrization of meso (1R,2S)‐cyclohex‐4‐ene‐1,2‐diol in an enantioselective and substrate directed fashion. The method provides all cis substituted highly functionalized chiral allylic alcohols in a complementary fashion to other Heck protocols.The products were obtained in high enantioselectivities (higher than 95% ee) and moderate to high yields (38–87%). The noncovalent interactions responsible for the directing effect were elucidated through computational examination of relevant minima and transition structures.
中文翻译:

非共价底物定向的顺式环己烯-4-烯-1-二醇的对映选择性Heck不对称化:所有顺式手性5-芳基-环己烯-3-烯-1,2-二醇的合成及机理研究
对映选择性底物定向的Heck反应对于复杂分子的立体选择性合成是理想的。但是,由于手性配体和导向基团的配位要求,这种方法学尚不完善。我们在此报告的desymmetrization内消旋(1 - [R,2小号) -环己-4-烯-1,2-二醇在对映选择性和基底定向的方式。该方法提供所有顺式取代了其他Heck方案的互补方式。该产品以高对映选择性(高于95%ee)和中等至高收率(38-87%)获得。通过相关最小和过渡结构的计算检查,阐明了负责指导作用的非共价相互作用。
更新日期:2018-08-14
中文翻译:

非共价底物定向的顺式环己烯-4-烯-1-二醇的对映选择性Heck不对称化:所有顺式手性5-芳基-环己烯-3-烯-1,2-二醇的合成及机理研究
对映选择性底物定向的Heck反应对于复杂分子的立体选择性合成是理想的。但是,由于手性配体和导向基团的配位要求,这种方法学尚不完善。我们在此报告的desymmetrization内消旋(1 - [R,2小号) -环己-4-烯-1,2-二醇在对映选择性和基底定向的方式。该方法提供所有顺式取代了其他Heck方案的互补方式。该产品以高对映选择性(高于95%ee)和中等至高收率(38-87%)获得。通过相关最小和过渡结构的计算检查,阐明了负责指导作用的非共价相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号