当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iron(iii)porphyrin electrocatalyzed enantioselective carbon-chloride bond cleavage of hexachlorocyclohexanes (HCHs): combined experimental investigation and theoretical calculations†
Dalton Transactions ( IF 4 ) Pub Date : 2018-07-23 00:00:00 , DOI: 10.1039/c8dt02510j Xu Liang 1, 2, 3, 4, 5 , Minzhi Li 1, 2, 3, 4 , John Mack 6, 7, 8 , Kevin Lobb 6, 7, 8 , Weihua Zhu 1, 2, 3, 4, 5
Dalton Transactions ( IF 4 ) Pub Date : 2018-07-23 00:00:00 , DOI: 10.1039/c8dt02510j Xu Liang 1, 2, 3, 4, 5 , Minzhi Li 1, 2, 3, 4 , John Mack 6, 7, 8 , Kevin Lobb 6, 7, 8 , Weihua Zhu 1, 2, 3, 4, 5
Affiliation
|
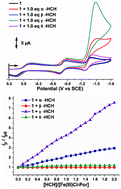
Enantioselective electrocatalysis of α-, β-, γ- and δ-hexachlorocyclohexanes (HCHs) by tetrakis-pentafluorophenyl-Fe(III)porphyrin is described. The first example of the combined use of electrochemical measurements and theoretical calculations to determine the mechanism of the enantioselective C–Cl bond cleavage of the electrocatalysis is reported. The electrochemical measurements demonstrate that the reactivity of the HCHs follows the order γ-HCH > α-HCH > δ-HCH > β-HCH. Steric considerations and a molecular orbital theory approach can be used to rationalize the enantioselective nature of the catalysis based on the ease of approach of each Cl atom to the central Fe(I) ion and a consideration of the nodes on the C–Cl bonds that weaken these bonds in a manner that results in bond cleavage and the formation of an Fe–Cl bond.
中文翻译:

铁(iii)卟啉电催化六氯环己烷(HCHs)的对映选择性碳-氯键裂解:结合实验研究和理论计算†
描述了四-五氟苯基-Fe(III)卟啉对α-,β-,γ-和δ-六氯环己烷(HCHs)的对映选择性电催化。报道了结合使用电化学测量和理论计算来确定电催化对映选择性C–Cl键断裂机理的第一个例子。电化学测量表明,六氯环己烷的反应性遵循γ-HCH>α-HCH>δ-HCH>β-HCH的顺序。立体考虑和分子轨道理论方法可用于基于每个Cl原子接近中心Fe的难易程度合理化催化的对映选择性(I离子和考虑到C–Cl键上的节点会削弱这些键,从而导致键断裂并形成Fe–Cl键。
更新日期:2018-07-23
中文翻译:

铁(iii)卟啉电催化六氯环己烷(HCHs)的对映选择性碳-氯键裂解:结合实验研究和理论计算†
描述了四-五氟苯基-Fe(III)卟啉对α-,β-,γ-和δ-六氯环己烷(HCHs)的对映选择性电催化。报道了结合使用电化学测量和理论计算来确定电催化对映选择性C–Cl键断裂机理的第一个例子。电化学测量表明,六氯环己烷的反应性遵循γ-HCH>α-HCH>δ-HCH>β-HCH的顺序。立体考虑和分子轨道理论方法可用于基于每个Cl原子接近中心Fe的难易程度合理化催化的对映选择性(I离子和考虑到C–Cl键上的节点会削弱这些键,从而导致键断裂并形成Fe–Cl键。



























 京公网安备 11010802027423号
京公网安备 11010802027423号