当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lattice Engineering on Metal Cocatalysts for Enhanced Photocatalytic Reduction of CO2 into CH4
ChemSusChem ( IF 8.4 ) Pub Date : 2018-08-30 , DOI: 10.1002/cssc.201801294 Leihong Zhao 1 , Fan Ye 1 , Dongmei Wang 1 , Xiaotong Cai 1 , Chenchen Meng 1 , Hanshi Xie 1 , Jiali Zhang 1 , Song Bai 1, 2
ChemSusChem ( IF 8.4 ) Pub Date : 2018-08-30 , DOI: 10.1002/cssc.201801294 Leihong Zhao 1 , Fan Ye 1 , Dongmei Wang 1 , Xiaotong Cai 1 , Chenchen Meng 1 , Hanshi Xie 1 , Jiali Zhang 1 , Song Bai 1, 2
Affiliation
|
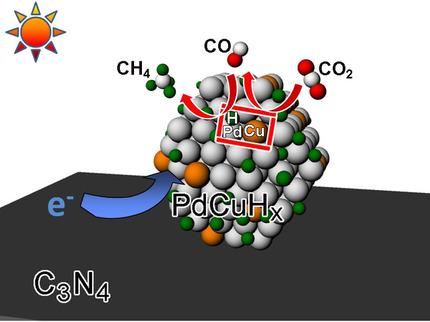
Photocatalytic conversion of CO2 into CH4 represents an appealing approach to alleviate the world's continued reliance on fossil fuels and global warming resulting from increasing CO2 concentrations in the atmosphere. However, its practical application is greatly limited by serious electron–hole recombination in the photocatalysts and the production of CO and H2 as side reactions. Herein, for the first time, it is demonstrated that the photocatalytic reduction of CO2 to CH4 can be significantly improved through the simultaneous alloying and hydriding of metal cocatalysts. The isolation of Cu and H atoms in Pd lattices play three roles in the enhancement of CO2 to CH4 conversion: 1) Cu atoms provide catalytic sites to reduce CO2 into CO and then to CH4 to suppress H2 evolution; 2) H atoms improve the electron‐trapping ability of cocatalysts; and 3) H atoms accelerate the reduction of CO to CH4, which is the rate‐limiting procedure in the conversion of CO2 into CH4. Arising from the synergistic interplay between Pd−H and Cu−CO sites, C3N4−Pd9Cu1Hx (15 mg) achieves 100 % selectivity for CH4 production with an average rate of 0.018 μmol h−1 under visible‐light irradiation. This work provides insights into the design of a cocatalyst for highly selective CO2 conversion through lattice engineering at atomic precision.
中文翻译:

金属助催化剂的晶格工程,用于增强光催化将CO2还原为CH4
将CO 2光催化转化为CH 4代表了一种吸引人的方法,可以减轻世界上对大气中CO 2浓度不断增加所导致的对化石燃料和全球变暖的持续依赖。但是,其实际应用受到光催化剂中严重的电子-空穴复合以及副反应的产生CO和H 2的极大限制。在此,首次证明通过金属助催化剂的同时合金化和氢化,可以显着改善CO 2向CH 4的光催化还原。Pd晶格中Cu和H原子的隔离在增强CO 2中起三个作用CH 4转化:1)Cu原子提供催化位点,将CO 2还原为CO,然后提供CH 4抑制H 2的释放;2)H原子提高了助催化剂的电子俘获能力;3)H原子加速了CO向CH 4的还原,这是CO 2转化为CH 4的限速过程。由于Pd-H和Cu-CO位点之间的协同相互作用,C 3 N 4 -Pd 9 Cu 1 H x(15 mg)对CH 4的生产具有100%的选择性,平均速率为0.018μmolh -1在可见光照射下。这项工作提供了对通过原子精度的晶格工程进行高选择性CO 2转化的助催化剂设计的见解。
更新日期:2018-08-30
中文翻译:

金属助催化剂的晶格工程,用于增强光催化将CO2还原为CH4
将CO 2光催化转化为CH 4代表了一种吸引人的方法,可以减轻世界上对大气中CO 2浓度不断增加所导致的对化石燃料和全球变暖的持续依赖。但是,其实际应用受到光催化剂中严重的电子-空穴复合以及副反应的产生CO和H 2的极大限制。在此,首次证明通过金属助催化剂的同时合金化和氢化,可以显着改善CO 2向CH 4的光催化还原。Pd晶格中Cu和H原子的隔离在增强CO 2中起三个作用CH 4转化:1)Cu原子提供催化位点,将CO 2还原为CO,然后提供CH 4抑制H 2的释放;2)H原子提高了助催化剂的电子俘获能力;3)H原子加速了CO向CH 4的还原,这是CO 2转化为CH 4的限速过程。由于Pd-H和Cu-CO位点之间的协同相互作用,C 3 N 4 -Pd 9 Cu 1 H x(15 mg)对CH 4的生产具有100%的选择性,平均速率为0.018μmolh -1在可见光照射下。这项工作提供了对通过原子精度的晶格工程进行高选择性CO 2转化的助催化剂设计的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号