Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facile synthesis of a hollow Ni–Fe–B nanochain and its enhanced catalytic activity for hydrogen generation from NaBH4 hydrolysis†
RSC Advances ( IF 3.9 ) Pub Date : 2018-07-19 00:00:00 , DOI: 10.1039/c8ra03848a Jie Guo 1 , Yongjiang Hou 1 , Bo Li 1
RSC Advances ( IF 3.9 ) Pub Date : 2018-07-19 00:00:00 , DOI: 10.1039/c8ra03848a Jie Guo 1 , Yongjiang Hou 1 , Bo Li 1
Affiliation
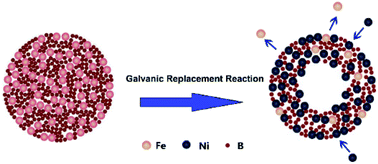
|
A hollow Ni–Fe–B nanochain is successfully synthesized by a galvanic replacement method using a Fe–B nanocomposite and a NiCl2 solution as the template and additional reagent, respectively. Both the concentration of Ni and the morphology of the resulting Ni–Fe–B alloy are controlled by varying the duration of the replacement process during the synthesis. The Ni–Fe–B sample synthesized for 60 min (Ni–Fe–B-60) shows the best catalytic activity at 313 K, with a hydrogen production rate of 4320 mL min−1 gcat−1 and an activation energy for the NaBH4 hydrolysis reaction of 33.7 kJ mol−1. The good performance of Ni–Fe–B-60 towards the hydrolysis of NaBH4 can be ascribed to both hollow nanochain structural and electronic effects. Furthermore, the effects of temperature, catalyst amount, and concentration of NaOH and NaBH4 on the hydrolysis process are systematically studied, and an overall kinetic rate equation is obtained. The hollow Ni–Fe–B nanochain catalyst also shows good reusability characteristics and maintained its initial activity after 5 consecutive cycles.
中文翻译:

空心 Ni-Fe-B 纳米链的简便合成及其增强的 NaBH4 水解产氢催化活性†
分别使用Fe-B纳米复合材料和NiCl 2溶液作为模板和附加试剂,通过电取代法成功合成了空心Ni-Fe-B纳米链。Ni 的浓度和所得 Ni-Fe-B 合金的形貌都是通过改变合成过程中置换过程的持续时间来控制的。合成60分钟的Ni-Fe-B样品(Ni-Fe-B-60)在313 K时表现出最佳的催化活性,产氢率为4320 mL min −1 g cat −1 ,活化能为33.7 kJ mol -1的NaBH 4水解反应。Ni-Fe-B-60 对 NaBH 4水解的良好性能可归因于中空纳米链结构和电子效应。进一步系统研究了温度、催化剂用量、NaOH和NaBH 4浓度对水解过程的影响,得到了总体动力学速率方程。空心Ni-Fe-B纳米链催化剂还表现出良好的可重复使用特性,并且在连续5次循环后仍保持其初始活性。
更新日期:2018-07-19
中文翻译:

空心 Ni-Fe-B 纳米链的简便合成及其增强的 NaBH4 水解产氢催化活性†
分别使用Fe-B纳米复合材料和NiCl 2溶液作为模板和附加试剂,通过电取代法成功合成了空心Ni-Fe-B纳米链。Ni 的浓度和所得 Ni-Fe-B 合金的形貌都是通过改变合成过程中置换过程的持续时间来控制的。合成60分钟的Ni-Fe-B样品(Ni-Fe-B-60)在313 K时表现出最佳的催化活性,产氢率为4320 mL min −1 g cat −1 ,活化能为33.7 kJ mol -1的NaBH 4水解反应。Ni-Fe-B-60 对 NaBH 4水解的良好性能可归因于中空纳米链结构和电子效应。进一步系统研究了温度、催化剂用量、NaOH和NaBH 4浓度对水解过程的影响,得到了总体动力学速率方程。空心Ni-Fe-B纳米链催化剂还表现出良好的可重复使用特性,并且在连续5次循环后仍保持其初始活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号