当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Conformational Free Energy Changes via an Alchemical Path without Reaction Coordinates
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-07-19 00:00:00 , DOI: 10.1021/acs.jpclett.8b01851 Peng He 1 , Bin W Zhang 1 , Shima Arasteh 1 , Lingle Wang 2 , Robert Abel 2 , Ronald M Levy 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-07-19 00:00:00 , DOI: 10.1021/acs.jpclett.8b01851 Peng He 1 , Bin W Zhang 1 , Shima Arasteh 1 , Lingle Wang 2 , Robert Abel 2 , Ronald M Levy 1
Affiliation
|
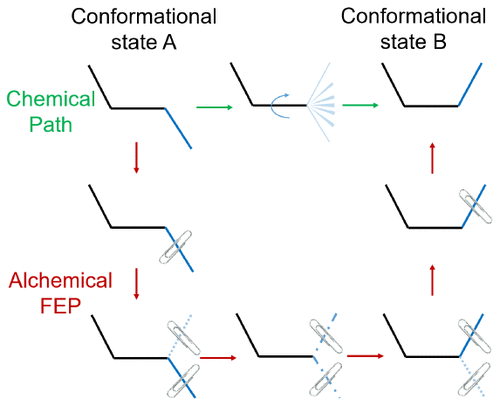
We introduce a novel method called restrain–free energy perturbation–release (R-FEP-R) to estimate conformational free energy changes via an alchemical path, which for some conformational landscapes like those associated with cellular signaling proteins in the kinase family is more direct and readily converged than the corresponding free energy changes along the physical path. The R-FEP-R method was developed from the dual topology free energy perturbation method that is widely applied to estimate the binding free energy difference between two ligands. In R-FEP-R, the free energy change between two conformational basins is calculated by free energy perturbations that remove those atoms involved in the conformational change from their initial conformational basin while simultaneously growing them back according to the final conformational basin. Both the initial and final dual topology states are unphysical, but they are designed in a way such that the unphysical contributions to the initial and final partition functions cancel. Compared with other advanced sampling algorithms such as umbrella sampling and metadynamics, the R-FEP-R method does not require predetermined transition pathways or reaction coordinates that connect the two conformational states. As a first illustration, the R-FEP-R method was applied to calculate the free energy change between conformational basins for alanine dipeptide in solution and for a side chain in the binding pocket of T4 lysozyme. The results obtained by R-FEP-R agree with the benchmarks very well.
中文翻译:

通过炼金路径改变构象自由能,无需反应坐标
我们引入了一种称为自由抑制能量扰动释放(R-FEP-R)的新方法,通过炼金术路径估计构象自由能变化,这对于某些构象景观(例如与激酶家族中的细胞信号蛋白相关的构象景观)来说更直接并且比相应的自由能沿物理路径的变化更容易收敛。R-FEP-R 方法是从双拓扑自由能微扰方法发展而来的,该方法广泛应用于估计两个配体之间的结合自由能差异。在R-FEP-R中,两个构象盆之间的自由能变化是通过自由能扰动来计算的,自由能扰动将那些参与构象变化的原子从其初始构象盆中移除,同时根据最终构象盆将它们生长回来。初始和最终的对偶拓扑状态都是非物理的,但它们的设计方式使得对初始和最终配分函数的非物理贡献相互抵消。与伞式采样和元动力学等其他先进采样算法相比,R-FEP-R方法不需要预先确定连接两种构象状态的转变路径或反应坐标。作为第一个说明,R-FEP-R 方法用于计算溶液中丙氨酸二肽的构象盆和 T4 溶菌酶结合袋中的侧链之间的自由能变化。R-FEP-R 获得的结果与基准非常吻合。
更新日期:2018-07-19
中文翻译:

通过炼金路径改变构象自由能,无需反应坐标
我们引入了一种称为自由抑制能量扰动释放(R-FEP-R)的新方法,通过炼金术路径估计构象自由能变化,这对于某些构象景观(例如与激酶家族中的细胞信号蛋白相关的构象景观)来说更直接并且比相应的自由能沿物理路径的变化更容易收敛。R-FEP-R 方法是从双拓扑自由能微扰方法发展而来的,该方法广泛应用于估计两个配体之间的结合自由能差异。在R-FEP-R中,两个构象盆之间的自由能变化是通过自由能扰动来计算的,自由能扰动将那些参与构象变化的原子从其初始构象盆中移除,同时根据最终构象盆将它们生长回来。初始和最终的对偶拓扑状态都是非物理的,但它们的设计方式使得对初始和最终配分函数的非物理贡献相互抵消。与伞式采样和元动力学等其他先进采样算法相比,R-FEP-R方法不需要预先确定连接两种构象状态的转变路径或反应坐标。作为第一个说明,R-FEP-R 方法用于计算溶液中丙氨酸二肽的构象盆和 T4 溶菌酶结合袋中的侧链之间的自由能变化。R-FEP-R 获得的结果与基准非常吻合。


























 京公网安备 11010802027423号
京公网安备 11010802027423号