Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Accumulation of succinate controls activation of adipose tissue thermogenesis
Nature ( IF 64.8 ) Pub Date : 2018-07-18 , DOI: 10.1038/s41586-018-0353-2 Evanna L Mills 1, 2 , Kerry A Pierce 3 , Mark P Jedrychowski 1, 2 , Ryan Garrity 1 , Sally Winther 1, 2 , Sara Vidoni 1, 2 , Takeshi Yoneshiro 4 , Jessica B Spinelli 2 , Gina Z Lu 1 , Lawrence Kazak 5 , Alexander S Banks 6 , Marcia C Haigis 2 , Shingo Kajimura 4 , Michael P Murphy 7 , Steven P Gygi 2 , Clary B Clish 3 , Edward T Chouchani 1, 2
Nature ( IF 64.8 ) Pub Date : 2018-07-18 , DOI: 10.1038/s41586-018-0353-2 Evanna L Mills 1, 2 , Kerry A Pierce 3 , Mark P Jedrychowski 1, 2 , Ryan Garrity 1 , Sally Winther 1, 2 , Sara Vidoni 1, 2 , Takeshi Yoneshiro 4 , Jessica B Spinelli 2 , Gina Z Lu 1 , Lawrence Kazak 5 , Alexander S Banks 6 , Marcia C Haigis 2 , Shingo Kajimura 4 , Michael P Murphy 7 , Steven P Gygi 2 , Clary B Clish 3 , Edward T Chouchani 1, 2
Affiliation
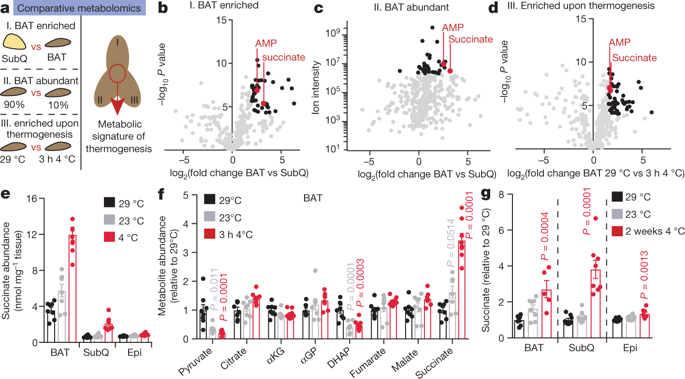
|
Thermogenesis by brown and beige adipose tissue, which requires activation by external stimuli, can counter metabolic disease1. Thermogenic respiration is initiated by adipocyte lipolysis through cyclic AMP–protein kinase A signalling; this pathway has been subject to longstanding clinical investigation2–4. Here we apply a comparative metabolomics approach and identify an independent metabolic pathway that controls acute activation of adipose tissue thermogenesis in vivo. We show that substantial and selective accumulation of the tricarboxylic acid cycle intermediate succinate is a metabolic signature of adipose tissue thermogenesis upon activation by exposure to cold. Succinate accumulation occurs independently of adrenergic signalling, and is sufficient to elevate thermogenic respiration in brown adipocytes. Selective accumulation of succinate may be driven by a capacity of brown adipocytes to sequester elevated circulating succinate. Furthermore, brown adipose tissue thermogenesis can be initiated by systemic administration of succinate in mice. Succinate from the extracellular milieu is rapidly metabolized by brown adipocytes, and its oxidation by succinate dehydrogenase is required for activation of thermogenesis. We identify a mechanism whereby succinate dehydrogenase-mediated oxidation of succinate initiates production of reactive oxygen species, and drives thermogenic respiration, whereas inhibition of succinate dehydrogenase supresses thermogenesis. Finally, we show that pharmacological elevation of circulating succinate drives UCP1-dependent thermogenesis by brown adipose tissue in vivo, which stimulates robust protection against diet-induced obesity and improves glucose tolerance. These findings reveal an unexpected mechanism for control of thermogenesis, using succinate as a systemically-derived thermogenic molecule.A comparative metabolomics approach is used to identify succinate as a key activator of thermogenesis in brown adipose tissue.
中文翻译:

琥珀酸的积累控制脂肪组织产热的激活
棕色和米色脂肪组织的产热需要外部刺激激活,可以对抗代谢疾病1。产热呼吸由脂肪细胞脂解通过环 AMP-蛋白激酶 A 信号启动;该途径已接受长期的临床研究2-4。在这里,我们应用比较代谢组学方法并确定了控制体内脂肪组织产热急性激活的独立代谢途径。我们表明,三羧酸循环中间体琥珀酸的大量和选择性积累是脂肪组织在暴露于冷时激活后产热的代谢特征。琥珀酸积累独立于肾上腺素能信号传导,并足以提高棕色脂肪细胞的产热呼吸。琥珀酸的选择性积累可能是由棕色脂肪细胞隔离升高的循环琥珀酸的能力驱动的。此外,棕色脂肪组织产热可以通过在小鼠中全身施用琥珀酸盐来启动。来自细胞外环境的琥珀酸被棕色脂肪细胞快速代谢,琥珀酸脱氢酶的氧化是产热激活所必需的。我们确定了一种机制,即琥珀酸脱氢酶介导的琥珀酸氧化引发活性氧物质的产生,并驱动产热呼吸,而抑制琥珀酸脱氢酶则抑制产热。最后,我们表明循环琥珀酸盐的药理学升高通过体内棕色脂肪组织驱动 UCP1 依赖性产热,它刺激了对饮食引起的肥胖的强大保护,并提高了葡萄糖耐量。这些发现揭示了一种意想不到的控制产热的机制,使用琥珀酸作为系统衍生的产热分子。比较代谢组学方法用于鉴定琥珀酸是棕色脂肪组织产热的关键激活剂。
更新日期:2018-07-18
中文翻译:

琥珀酸的积累控制脂肪组织产热的激活
棕色和米色脂肪组织的产热需要外部刺激激活,可以对抗代谢疾病1。产热呼吸由脂肪细胞脂解通过环 AMP-蛋白激酶 A 信号启动;该途径已接受长期的临床研究2-4。在这里,我们应用比较代谢组学方法并确定了控制体内脂肪组织产热急性激活的独立代谢途径。我们表明,三羧酸循环中间体琥珀酸的大量和选择性积累是脂肪组织在暴露于冷时激活后产热的代谢特征。琥珀酸积累独立于肾上腺素能信号传导,并足以提高棕色脂肪细胞的产热呼吸。琥珀酸的选择性积累可能是由棕色脂肪细胞隔离升高的循环琥珀酸的能力驱动的。此外,棕色脂肪组织产热可以通过在小鼠中全身施用琥珀酸盐来启动。来自细胞外环境的琥珀酸被棕色脂肪细胞快速代谢,琥珀酸脱氢酶的氧化是产热激活所必需的。我们确定了一种机制,即琥珀酸脱氢酶介导的琥珀酸氧化引发活性氧物质的产生,并驱动产热呼吸,而抑制琥珀酸脱氢酶则抑制产热。最后,我们表明循环琥珀酸盐的药理学升高通过体内棕色脂肪组织驱动 UCP1 依赖性产热,它刺激了对饮食引起的肥胖的强大保护,并提高了葡萄糖耐量。这些发现揭示了一种意想不到的控制产热的机制,使用琥珀酸作为系统衍生的产热分子。比较代谢组学方法用于鉴定琥珀酸是棕色脂肪组织产热的关键激活剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号