当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selection between Diastereomeric Kinetic vs Thermodynamic Carbonyl Binding Modes Enables Enantioselective Iridium-Catalyzed anti-(α-Aryl)allylation of Aqueous Fluoral Hydrate and Difluoroacetaldehyde Ethyl Hemiacetal
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-07-18 , DOI: 10.1021/jacs.8b05725 James M. Cabrera 1 , Johannes Tauber 1 , Wandi Zhang 1 , Ming Xiang 1 , Michael J. Krische 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-07-18 , DOI: 10.1021/jacs.8b05725 James M. Cabrera 1 , Johannes Tauber 1 , Wandi Zhang 1 , Ming Xiang 1 , Michael J. Krische 1
Affiliation
|
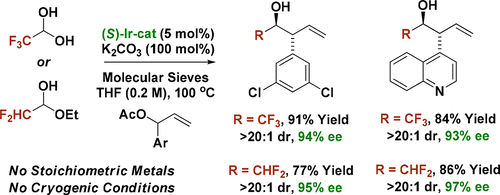
Enantioselectivity increases with increasing carbonyl electrophilicity in 2-propanol-mediated reductive couplings of aldehydes with branched aryl-substituted allylic acetates to form products of carbonyl anti-(α-aryl)allylation. This unusual phenomenon is caused by aldehyde coordination to diastereomeric kinetic vs thermodynamic carbonyl binding sites that deliver enantiomeric products. Exploiting this effect, anti-diastereo- and enantioselective (α-aryl)allylations of fluoral hydrate and difluoroacetaldehyde ethyl hemiacetal were developed.
中文翻译:

非对映体动力学与热力学羰基结合模式之间的选择使氟水合物水溶液和二氟乙醛乙基半缩醛的对映选择性铱催化抗(α-芳基)烯丙基化成为可能
在 2-丙醇介导的醛与支链芳基取代的烯丙基乙酸酯的还原偶联中,随着羰基亲电性的增加,对映选择性增加,以形成羰基抗(α-芳基)烯丙基化的产物。这种不寻常的现象是由醛与非对映体动力学与提供对映体产物的热力学羰基结合位点的配位引起的。利用这种效应,开发了氟水合物和二氟乙醛乙基半缩醛的抗非对映选择性和对映选择性(α-芳基)烯丙基化。
更新日期:2018-07-18
中文翻译:

非对映体动力学与热力学羰基结合模式之间的选择使氟水合物水溶液和二氟乙醛乙基半缩醛的对映选择性铱催化抗(α-芳基)烯丙基化成为可能
在 2-丙醇介导的醛与支链芳基取代的烯丙基乙酸酯的还原偶联中,随着羰基亲电性的增加,对映选择性增加,以形成羰基抗(α-芳基)烯丙基化的产物。这种不寻常的现象是由醛与非对映体动力学与提供对映体产物的热力学羰基结合位点的配位引起的。利用这种效应,开发了氟水合物和二氟乙醛乙基半缩醛的抗非对映选择性和对映选择性(α-芳基)烯丙基化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号