当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Organocatalytic Synthesis of gem‐Difluorinated C2‐Spiro Indolines and Pyrimido[1,2‐a]benzimidazoles from 2‐Alkynyl‐3,3‐Difluoro‐3H‐Indoles
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-08-03 , DOI: 10.1002/adsc.201800716 Xiang-Yu Mao 1 , Xiao-Tong Lin 1 , Meng Yang 1 , Guo-Shu Chen 1 , Yun-Lin Liu 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-08-03 , DOI: 10.1002/adsc.201800716 Xiang-Yu Mao 1 , Xiao-Tong Lin 1 , Meng Yang 1 , Guo-Shu Chen 1 , Yun-Lin Liu 1
Affiliation
|
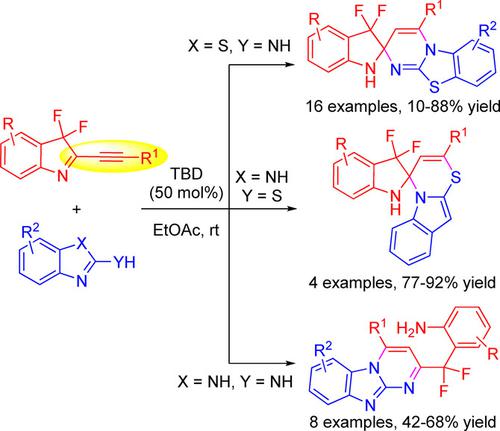
In this communication, we report a substrate‐controlled product diversity in the 1,5,7‐triazabicyclo[4.4.0]dec‐5‐ene (TBD)‐catalyzed cascade cyclization of 2‐alkynyl‐3,3‐difluoro‐3H‐indoles with N,N‐ or N,S‐bis‐nucleophiles. The products represent two important heterocyclic compounds, C2‐spiro indoline, and pyrimido[1,2‐a]benzimidazole, featuring a versatile gem‐difluoromethylene group on the framework. In these cascade processes, two new C−N bonds, or one C−S and one C−N bond, are consecutively formed in a single step. The present protocol is characterized with high regioselectivity, high yield, broad substrate scope, good functional group tolerance, facile scalability and mild reaction conditions.
中文翻译:

从2-炔基-3,3-二氟-3H-吲哚类化合物催化合成宝石二氟化C2-螺二氢吲哚和嘧啶并[1,2-a]苯并咪唑
在本交流中,我们报告了在1,5,7-三氮杂双环[4.4.0] dec-5-烯(TBD)催化的2-炔基-3,3-二氟-3级联环化反应中,受基质控制的产品多样性具有N,N-或N,S-双亲核试剂的H吲哚。产品代表两个重要的杂环化合物,C2-螺吲哚啉和嘧啶并[1,2- a ]苯并咪唑,在骨架上具有通用的宝石-二氟亚甲基基团。在这些级联过程中,两个新的CN键或一个CS和一个CN键在一个步骤中连续形成。本协议的特点是区域选择性高,收率高,底物范围广,官能团耐受性好,可扩展性和温和的反应条件。
更新日期:2018-08-03
中文翻译:

从2-炔基-3,3-二氟-3H-吲哚类化合物催化合成宝石二氟化C2-螺二氢吲哚和嘧啶并[1,2-a]苯并咪唑
在本交流中,我们报告了在1,5,7-三氮杂双环[4.4.0] dec-5-烯(TBD)催化的2-炔基-3,3-二氟-3级联环化反应中,受基质控制的产品多样性具有N,N-或N,S-双亲核试剂的H吲哚。产品代表两个重要的杂环化合物,C2-螺吲哚啉和嘧啶并[1,2- a ]苯并咪唑,在骨架上具有通用的宝石-二氟亚甲基基团。在这些级联过程中,两个新的CN键或一个CS和一个CN键在一个步骤中连续形成。本协议的特点是区域选择性高,收率高,底物范围广,官能团耐受性好,可扩展性和温和的反应条件。



























 京公网安备 11010802027423号
京公网安备 11010802027423号