当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Supramolecular propensity of suckerin proteins is driven by β-sheets and aromatic interactions as revealed by solution NMR†
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-07-16 00:00:00 , DOI: 10.1039/c8bm00556g Akshita Kumar 1, 2, 3, 4 , Harini Mohanram 1, 2, 3, 4 , Kiat Whye Kong 5, 6, 7, 8, 9 , Rubayn Goh 5, 6, 7, 8, 9 , Shawn Hoon 5, 6, 7, 8, 9 , Julien Lescar 10, 11, 12, 13, 14 , Ali Miserez 1, 2, 3, 4, 10
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-07-16 00:00:00 , DOI: 10.1039/c8bm00556g Akshita Kumar 1, 2, 3, 4 , Harini Mohanram 1, 2, 3, 4 , Kiat Whye Kong 5, 6, 7, 8, 9 , Rubayn Goh 5, 6, 7, 8, 9 , Shawn Hoon 5, 6, 7, 8, 9 , Julien Lescar 10, 11, 12, 13, 14 , Ali Miserez 1, 2, 3, 4, 10
Affiliation
|
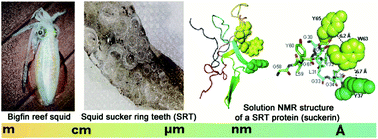
Suckerin proteins are a family of block co-polymer-like structural proteins that self-assemble into robust supramolecular structures – the sucker ring teeth (SRT) – which are located on the arms and tentacles of cephalopods and used to firmly capture preys. Suckerins are promising biomimetic protein-based biopolymers, but the supramolecular interactions stabilizing SRT remain unknown. Here, we report multi-dimensional Nuclear Magnetic Resonance (NMR) spectroscopy structural studies of an engineered suckerin protein composed of two main sequence modules. The protein adopts a dynamic structure with regions in both module 1 (M1: residues A42–A52) and module 2 (M2: residues G30–Y37 and G58–Y65) folding into anti-parallel β-sheets and displaying β-strand propensity, respectively. The obtained structure highlights that aromatic residues present in glycine (Gly)-rich M2 modules are involved in π–π stacking interactions, leading to the stabilization of the structural core. In addition, hydrogen/deuterium (H/D) exchange studies demonstrate a high protection of residues involved in intra-molecular β-sheets. Gaining a better understanding of the molecular structure of suckerin provides key molecular lessons that may be mimicked in the de novo design of peptide- and protein-based biomaterials with applications in medicine, tissue engineering and nanotechnology.
中文翻译:

溶液NMR显示,suckerin蛋白的超分子倾向受β-折叠和芳香族相互作用的驱动†
Suckerin蛋白是一组类似嵌段共聚物的结构蛋白,可以自组装成坚固的超分子结构-环形环齿(SRT),位于头足类动物的臂和触手上,用于牢固地捕获猎物。Suckerins是有前途的基于仿生蛋白质的生物聚合物,但稳定SRT的超分子相互作用仍然未知。在这里,我们报告了由两个主要序列模块组成的工程化的傻瓜蛋白的多维核磁共振(NMR)光谱结构研究。该蛋白质采用动态结构,在模块1(M1:残基A42–A52)和模块2(M2:残基G30–Y37和G58–Y65)中都折叠成反平行的β-折叠,并表现出β链倾向,分别。获得的结构突出表明,富含甘氨酸(Gly)的M2模块中存在的芳族残基参与π-π堆积相互作用,从而导致结构核的稳定。此外,氢/氘(H / D)交换研究表明,对分子内β-折叠中涉及的残基具有高度保护作用。更好地了解suckerin的分子结构提供了关键的分子课程,这些课程可能会被模仿。从头开始设计基于肽和蛋白质的生物材料,并将其应用于医学,组织工程和纳米技术。
更新日期:2018-07-16
中文翻译:

溶液NMR显示,suckerin蛋白的超分子倾向受β-折叠和芳香族相互作用的驱动†
Suckerin蛋白是一组类似嵌段共聚物的结构蛋白,可以自组装成坚固的超分子结构-环形环齿(SRT),位于头足类动物的臂和触手上,用于牢固地捕获猎物。Suckerins是有前途的基于仿生蛋白质的生物聚合物,但稳定SRT的超分子相互作用仍然未知。在这里,我们报告了由两个主要序列模块组成的工程化的傻瓜蛋白的多维核磁共振(NMR)光谱结构研究。该蛋白质采用动态结构,在模块1(M1:残基A42–A52)和模块2(M2:残基G30–Y37和G58–Y65)中都折叠成反平行的β-折叠,并表现出β链倾向,分别。获得的结构突出表明,富含甘氨酸(Gly)的M2模块中存在的芳族残基参与π-π堆积相互作用,从而导致结构核的稳定。此外,氢/氘(H / D)交换研究表明,对分子内β-折叠中涉及的残基具有高度保护作用。更好地了解suckerin的分子结构提供了关键的分子课程,这些课程可能会被模仿。从头开始设计基于肽和蛋白质的生物材料,并将其应用于医学,组织工程和纳米技术。



























 京公网安备 11010802027423号
京公网安备 11010802027423号