当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemical Transformation of Methanesulfonic Acid and Sodium Methanesulfonate through Heterogeneous OH Oxidation
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-07-17 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00072 Kai Chung Kwong 1 , Man Mei Chim 1 , Erik Hans Hoffmann 2 , Andreas Tilgner 2 , Hartmut Herrmann 2 , James F. Davies 3 , Kevin R. Wilson 4 , Man Nin Chan 1, 5
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-07-17 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00072 Kai Chung Kwong 1 , Man Mei Chim 1 , Erik Hans Hoffmann 2 , Andreas Tilgner 2 , Hartmut Herrmann 2 , James F. Davies 3 , Kevin R. Wilson 4 , Man Nin Chan 1, 5
Affiliation
|
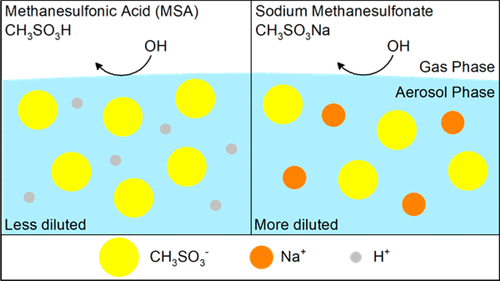
Methanesulfonic acid (CH3SO3H, MSA) is one of the major organosulfur acids formed from the photochemical oxidation of dimethyl sulfide (DMS) produced by oceanic phytoplankton. MSA can react with metal halides (e.g., sodium chloride) in ambient aerosols to form methanesulfonate salts (e.g., sodium methanesulfonate, CH3SO3Na). While the formation processes of MSA and its salts are reasonably well understood, their subsequent chemical transformations in the atmosphere are not fully resolved. MSA and its salts accumulate near the aerosol surface due to their surface activities, which make them available to heterogeneous oxidation at the gas–aerosol interface by oxidants such as hydroxyl (OH) radicals. In this work, the compositional changes of aerosol comprised of MSA and its sodium salt (CH3SO3Na) are measured following heterogeneous OH oxidation. An aerosol flow tube reactor is coupled with a soft atmospheric pressure ionization source (Direct Analysis in Real Time, DART) and a high-resolution mass spectrometer at a relative humidity (RH) of 90%. DART-aerosol mass spectra reveal that MSA and CH3SO3Na can be detected as methanesulfonate ion (CH3SO3–) with minimal fragmentation in the negative ionization mode. Kinetic measurements show that OH oxidation with MSA and CH3SO3Na has an effective OH uptake coefficient of 0.45 ± 0.14 and 0.20 ± 0.06, respectively, revealing that MSA reacts with OH radical faster than its sodium salt. One possibility for the difference in reactivity of these two compounds is that CH3SO3Na is more hygroscopic than MSA. The increase in the coverage of water molecules at the surface of CH3SO3Na might reduce the reactive collision probability between CH3SO3– and OH radicals, resulting in a smaller reaction rate. MSA and CH3SO3Na dissociate to form CH3SO3–, which tends to fragment into formaldehyde (HCHO) and a sulfite radical (SO3•–) upon oxidation. Formaldehyde partitions back to the gas phase owing to its high volatility, and SO3•– can initiate a series of chain reactions involving various inorganic sulfur radicals and ions in the aerosol phase. Overall, the fragmentation and SO3•–-initiated chemistry are the major processes controlling the chemical evolution of MSA and its sodium salt aerosols during heterogeneous OH oxidation.
中文翻译:

异构OH氧化甲烷磺酸和甲烷磺酸钠的化学转化。
甲磺酸(CH 3 SO 3 H,MSA)是由海洋浮游植物产生的二甲基硫醚(DMS)的光化学氧化形成的主要有机磺酸之一。MSA可以与周围的气溶胶中的金属卤化物(例如氯化钠)反应形成甲磺酸盐(例如甲磺酸钠,CH 3 SO 3Na)。虽然对MSA及其盐的形成过程有充分的了解,但它们随后在大气中的化学转化仍未完全解决。MSA及其盐由于其表面活性而在气溶胶表面附近积聚,这使得它们可通过氧化剂(例如羟基(OH))在气体-气溶胶界面处进行异质氧化。在这项工作中,在异质OH氧化后,测量了由MSA及其钠盐(CH 3 SO 3 Na)组成的气溶胶的成分变化。气溶胶流管反应器与软大气压电离源(实时实时直接分析,DART)和高分辨率质谱仪(相对湿度(RH)为90%)耦合。DART气溶胶质谱表明MSA和CH3 SO 3 Na可以检测为甲磺酸根离子(CH 3 SO 3 –),在负电离模式下碎片最少。动力学测量结果表明,用MSA和CH 3 SO 3 Na进行OH氧化时,其有效OH吸收系数分别为0.45±0.14和0.20±0.06,这表明MSA与OH自由基的反应比其钠盐的反应更快。这两种化合物的反应性不同的一种可能性是,CH 3 SO 3 Na的吸湿性比MSA高。CH 3 SO 3表面水分子覆盖率的增加钠可能会降低反应性碰撞概率CH之间3 SO 3 -和OH自由基,导致更小的反应速率。MSA和CH 3 SO 3 Na解离形成CH 3 SO 3 –,在氧化时趋于分解成甲醛(HCHO)和亚硫酸根(SO 3 •–)。由于甲醛的高挥发性,它会重新分配到气相中,因此SO 3 •可以引发一系列涉及气溶胶相中各种无机硫自由基和离子的链反应。总体而言,碎片化和SO 3 •–引发的化学反应是控制MSA及其钠盐气溶胶在非均相OH氧化过程中化学演化的主要过程。
更新日期:2018-07-17
中文翻译:

异构OH氧化甲烷磺酸和甲烷磺酸钠的化学转化。
甲磺酸(CH 3 SO 3 H,MSA)是由海洋浮游植物产生的二甲基硫醚(DMS)的光化学氧化形成的主要有机磺酸之一。MSA可以与周围的气溶胶中的金属卤化物(例如氯化钠)反应形成甲磺酸盐(例如甲磺酸钠,CH 3 SO 3Na)。虽然对MSA及其盐的形成过程有充分的了解,但它们随后在大气中的化学转化仍未完全解决。MSA及其盐由于其表面活性而在气溶胶表面附近积聚,这使得它们可通过氧化剂(例如羟基(OH))在气体-气溶胶界面处进行异质氧化。在这项工作中,在异质OH氧化后,测量了由MSA及其钠盐(CH 3 SO 3 Na)组成的气溶胶的成分变化。气溶胶流管反应器与软大气压电离源(实时实时直接分析,DART)和高分辨率质谱仪(相对湿度(RH)为90%)耦合。DART气溶胶质谱表明MSA和CH3 SO 3 Na可以检测为甲磺酸根离子(CH 3 SO 3 –),在负电离模式下碎片最少。动力学测量结果表明,用MSA和CH 3 SO 3 Na进行OH氧化时,其有效OH吸收系数分别为0.45±0.14和0.20±0.06,这表明MSA与OH自由基的反应比其钠盐的反应更快。这两种化合物的反应性不同的一种可能性是,CH 3 SO 3 Na的吸湿性比MSA高。CH 3 SO 3表面水分子覆盖率的增加钠可能会降低反应性碰撞概率CH之间3 SO 3 -和OH自由基,导致更小的反应速率。MSA和CH 3 SO 3 Na解离形成CH 3 SO 3 –,在氧化时趋于分解成甲醛(HCHO)和亚硫酸根(SO 3 •–)。由于甲醛的高挥发性,它会重新分配到气相中,因此SO 3 •可以引发一系列涉及气溶胶相中各种无机硫自由基和离子的链反应。总体而言,碎片化和SO 3 •–引发的化学反应是控制MSA及其钠盐气溶胶在非均相OH氧化过程中化学演化的主要过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号