当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nucleoside triphosphate cosubstrates control the substrate profile and efficiency of aminoglycoside 3′-O-phosphotransferase type IIa†
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-07-16 00:00:00 , DOI: 10.1039/c8md00234g Selina Y. L. Holbrook 1, 2, 3, 4, 5 , Matthew S. Gentry 3, 4, 5, 6, 7 , Oleg V. Tsodikov 1, 2, 3, 4, 5 , Sylvie Garneau-Tsodikova 1, 2, 3, 4, 5
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-07-16 00:00:00 , DOI: 10.1039/c8md00234g Selina Y. L. Holbrook 1, 2, 3, 4, 5 , Matthew S. Gentry 3, 4, 5, 6, 7 , Oleg V. Tsodikov 1, 2, 3, 4, 5 , Sylvie Garneau-Tsodikova 1, 2, 3, 4, 5
Affiliation
|
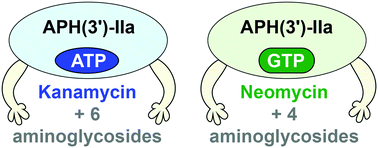
Aminoglycosides (AGs) are broad-spectrum antibiotics that play an important role in the control and treatment of bacterial infections. Despite the great antibacterial potency of AGs, resistance to these antibiotics has limited their clinical applications. The AG 3′-O-phosphotransferase of type IIa (APH(3′)-IIa) encoded by the neoR gene is a common bacterial AG resistance enzyme that inactivates AG antibiotics. This enzyme is used as a selection marker in molecular biology research. APH(3′)-IIa catalyzes the transfer of the γ-phosphoryl group of ATP to an AG at its 3′-OH group. Although APH(3′)-IIa has been reported to utilize exclusively ATP as a cosubstrate, we demonstrate that this enzyme can utilize a broad array of NTPs. By substrate profiling, TLC, and enzyme kinetics experiments, we probe AG phosphorylation by APH(3′)-IIa with an extensive panel of substrates and cosubstrates (13 AGs and 10 NTPs) for the purpose of gaining a thorough understanding of this resistance enzyme. We find, for the first time, that the identity of the NTP cosubstrate dictates the set of AGs modified by APH(3′)-IIa and the phosphorylation efficiency for different AGs.
中文翻译:

三磷酸核苷共底物控制的氨基糖苷类3'-衬底轮廓和效率ö磷酸转移酶IIa型†
氨基糖苷类(AGs)是广谱抗生素,在细菌感染的控制和治疗中起着重要作用。尽管AGs具有强大的抗菌效力,但对这些抗生素的抗药性限制了其临床应用。neo R编码的IIa型AG 3'- O-磷酸转移酶(APH(3')-IIa)基因是一种常见的细菌AG抗性酶,可以使AG抗生素失活。该酶在分子生物学研究中用作选择标记。APH(3')-IIa催化ATP的γ-磷酰基在其3'-OH基团处转移至AG。尽管已报道APH(3')-IIa仅利用ATP作为共底物,但我们证明该酶可以利用多种NTP。通过底物谱分析,TLC和酶动力学实验,我们通过广泛的底物和共底物面板(13个AG和10个NTP)来探测APH(3')-IIa引起的AG磷酸化,从而深入了解这种抗性酶。我们首次发现NTP共基质的身份决定了APH(3')-IIa修饰的AG的集合以及不同AG的磷酸化效率。
更新日期:2018-07-16
中文翻译:

三磷酸核苷共底物控制的氨基糖苷类3'-衬底轮廓和效率ö磷酸转移酶IIa型†
氨基糖苷类(AGs)是广谱抗生素,在细菌感染的控制和治疗中起着重要作用。尽管AGs具有强大的抗菌效力,但对这些抗生素的抗药性限制了其临床应用。neo R编码的IIa型AG 3'- O-磷酸转移酶(APH(3')-IIa)基因是一种常见的细菌AG抗性酶,可以使AG抗生素失活。该酶在分子生物学研究中用作选择标记。APH(3')-IIa催化ATP的γ-磷酰基在其3'-OH基团处转移至AG。尽管已报道APH(3')-IIa仅利用ATP作为共底物,但我们证明该酶可以利用多种NTP。通过底物谱分析,TLC和酶动力学实验,我们通过广泛的底物和共底物面板(13个AG和10个NTP)来探测APH(3')-IIa引起的AG磷酸化,从而深入了解这种抗性酶。我们首次发现NTP共基质的身份决定了APH(3')-IIa修饰的AG的集合以及不同AG的磷酸化效率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号