当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
ω‐Phthalimidoalkyl Aryl Ureas as Potent and Selective Inhibitors of Cholesterol Esterase
ChemMedChem ( IF 3.4 ) Pub Date : 2018-08-13 , DOI: 10.1002/cmdc.201800388 Florian M. Dato 1, 2 , Miriam Sheikh 1 , Rocky Z. Uhl 1 , Alexandra W. Schüller 1, 2 , Michaela Steinkrüger 1 , Peter Koch 1 , Jörg-Martin Neudörfl 2 , Michael Gütschow 3 , Bernd Goldfuss 2 , Markus Pietsch 1
ChemMedChem ( IF 3.4 ) Pub Date : 2018-08-13 , DOI: 10.1002/cmdc.201800388 Florian M. Dato 1, 2 , Miriam Sheikh 1 , Rocky Z. Uhl 1 , Alexandra W. Schüller 1, 2 , Michaela Steinkrüger 1 , Peter Koch 1 , Jörg-Martin Neudörfl 2 , Michael Gütschow 3 , Bernd Goldfuss 2 , Markus Pietsch 1
Affiliation
|
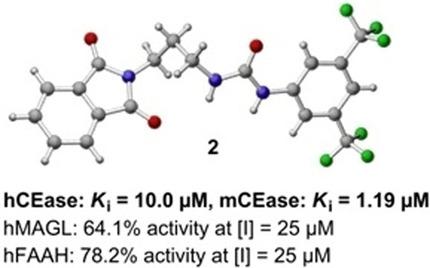
Cholesterol esterase (CEase), a serine hydrolase thought to be involved in atherogenesis and thus coronary heart disease, is considered as a target for inhibitor development. We investigated recombinant human and murine CEases with a new fluorometric assay in a structure–activity relationship study of a small library of ω‐phthalimidoalkyl aryl ureas. The urea motif with an attached 3,5‐bis(trifluoromethyl)phenyl group and the aromatic character of the ω‐phthalimide residue were most important for inhibitory activity. In addition, an alkyl chain composed of three or four methylene groups, connecting the urea and phthalimide moieties, was found to be an optimal spacer for inhibitors. The so‐optimized compounds 2 [1‐(3,5‐bis(trifluoromethyl)phenyl)‐3‐(3‐(1,3‐dioxoisoindolin‐2‐yl)propyl)urea] and 21 [1‐(3,5‐bis(trifluoromethyl)phenyl)‐3‐(4‐(1,3‐dioxoisoindolin‐2‐yl)butyl)urea] exhibited dissociation constants (Ki) of 1–19 μm on the two CEases and showed either a competitive (2 on the human enzyme and 21 on the murine enzyme) or a noncompetitive mode of inhibition. Two related serine hydrolases—monoacylglycerol lipase and fatty acid amide hydrolase—were inhibited by ω‐phthalimidoalkyl aryl ureas to a lesser extent.
中文翻译:

ω-邻苯二甲酰亚胺基烷基脲作为胆固醇酯酶的有效抑制剂和选择性抑制剂
胆固醇酯酶(CEase)是一种丝氨酸水解酶,被认为与动脉粥样硬化有关,因此与冠心病有关,被认为是抑制剂发展的目标。在一个小的ω-邻苯二甲酰亚胺基烷基芳基尿素文库的结构-活性关系研究中,我们用新的荧光测定法研究了重组人和鼠类CEase。带有3,5-双(三氟甲基)苯基的尿素基序和ω-邻苯二甲酰亚胺残基的芳香特性对于抑制活性最重要。另外,发现由三个或四个连接尿素和邻苯二甲酰亚胺部分的亚甲基组成的烷基链是抑制剂的最佳间隔基。如此优化的化合物2 [1-(3,5-双(三氟甲基)苯基)-3-(3-(1,3-二氧代异吲哚啉-2-基)丙基)脲]和21[1-(3,5-双(三氟甲基)苯基)-3-(4-(1,3- dioxoisoindolin -2-基)丁基)脲]显示解离常数(ķ我的1-19μ)米上的两种CEase并显示出竞争性抑制作用(对人类酶而言为2种,对鼠类酶而言为21种)或非竞争性抑制方式。ω-邻苯二甲酰亚胺基烷基芳基脲对二种相关的丝氨酸水解酶-单酰基甘油脂肪酶和脂肪酸酰胺水解酶的抑制程度较小。
更新日期:2018-08-13
中文翻译:

ω-邻苯二甲酰亚胺基烷基脲作为胆固醇酯酶的有效抑制剂和选择性抑制剂
胆固醇酯酶(CEase)是一种丝氨酸水解酶,被认为与动脉粥样硬化有关,因此与冠心病有关,被认为是抑制剂发展的目标。在一个小的ω-邻苯二甲酰亚胺基烷基芳基尿素文库的结构-活性关系研究中,我们用新的荧光测定法研究了重组人和鼠类CEase。带有3,5-双(三氟甲基)苯基的尿素基序和ω-邻苯二甲酰亚胺残基的芳香特性对于抑制活性最重要。另外,发现由三个或四个连接尿素和邻苯二甲酰亚胺部分的亚甲基组成的烷基链是抑制剂的最佳间隔基。如此优化的化合物2 [1-(3,5-双(三氟甲基)苯基)-3-(3-(1,3-二氧代异吲哚啉-2-基)丙基)脲]和21[1-(3,5-双(三氟甲基)苯基)-3-(4-(1,3- dioxoisoindolin -2-基)丁基)脲]显示解离常数(ķ我的1-19μ)米上的两种CEase并显示出竞争性抑制作用(对人类酶而言为2种,对鼠类酶而言为21种)或非竞争性抑制方式。ω-邻苯二甲酰亚胺基烷基芳基脲对二种相关的丝氨酸水解酶-单酰基甘油脂肪酶和脂肪酸酰胺水解酶的抑制程度较小。



























 京公网安备 11010802027423号
京公网安备 11010802027423号