当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Decoding the Rich Biological Properties of Noble Gases: How Well Can We Predict Noble Gas Binding to Diverse Proteins?
ChemMedChem ( IF 3.4 ) Pub Date : 2018-08-13 , DOI: 10.1002/cmdc.201800434 David A. Winkler 1, 2, 3 , Ira Katz 4, 5 , Géraldine Farjot 4 , Andrew C. Warden 6 , Aaron W. Thornton 1
ChemMedChem ( IF 3.4 ) Pub Date : 2018-08-13 , DOI: 10.1002/cmdc.201800434 David A. Winkler 1, 2, 3 , Ira Katz 4, 5 , Géraldine Farjot 4 , Andrew C. Warden 6 , Aaron W. Thornton 1
Affiliation
|
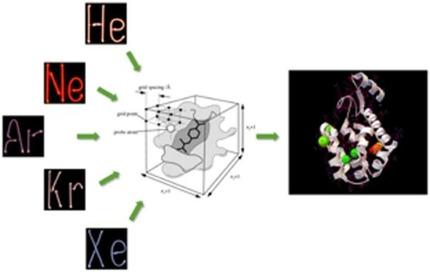
The chemically inert noble gases display a surprisingly rich spectrum of useful biological properties. Relatively little is known about the molecular mechanisms behind these effects. It is clearly not feasible to conduct large numbers of pharmacological experiments on noble gases to identify activity. Computational studies of the binding of noble gases and proteins can address this paucity of information and provide insight into mechanisms of action. We used bespoke computational grid calculations to predict the positions of energy minima in the interactions of noble gases with diverse proteins. The method was validated by quantifying how well simulations could predict binding positions in 131 diverse protein X‐ray structures containing 399 Xe and Kr atoms. We found excellent agreement between calculated and experimental binding positions of noble gases. 94 % of all crystallographic xenon atoms were within 1 Xe van der Waals (vdW) diameter of a predicted binding site, and 97 % lay within 2 vdW diameters. 100 % of crystallographic krypton atoms were within 1 Kr vdW diameter of a predicted binding site. We showed the feasibility of large‐scale computational screening of all ≈60 000 unique structures in the Protein Data Bank. This will elucidate biochemical mechanisms by which these novel ‘atomic drugs’ elicit their valuable biochemical properties and identify new medical uses.
中文翻译:

解码稀有气体的丰富生物特性:我们如何预测稀有气体与多种蛋白质的结合?
具有化学惰性的稀有气体显示出令人惊讶的丰富的有用生物学特性光谱。关于这些作用背后的分子机制知之甚少。在稀有气体上进行大量药理实验以鉴定活性显然是不可行的。稀有气体与蛋白质结合的计算研究可以解决这种信息匮乏的问题,并提供对作用机理的深入了解。我们使用了定制的计算网格计算来预测在稀有气体与各种蛋白质相互作用中能量极小值的位置。通过量化模拟如何很好地预测包含399个Xe和Kr原子的131种不同蛋白质X射线结构中的结合位置来验证该方法的有效性。我们发现稀有气体的计算结合位置与实验结合位置之间具有极好的一致性。所有晶体氙原子的94%在预测结合位点的1 Xe van der Waals(vdW)直径之内,而97%位于2 vdW直径之内。100%的结晶k原子在预测结合位点的1 Kr vdW直径内。我们展示了蛋白质数据库中所有≈60000个独特结构的大规模计算筛选的可行性。这将阐明生物化学机制,通过这些生物化学机制,这些新颖的“原子药物”会激发其宝贵的生物化学特性并确定新的医学用途。100%的结晶k原子在预测结合位点的1 Kr vdW直径内。我们展示了蛋白质数据库中所有≈60000个独特结构的大规模计算筛选的可行性。这将阐明生化机制,通过这些生化机制,这些新颖的“原子药物”会激发其宝贵的生化特性并确定新的医学用途。100%的结晶k原子在预测结合位点的1 Kr vdW直径内。我们展示了蛋白质数据库中所有≈60000个独特结构的大规模计算筛选的可行性。这将阐明生化机制,通过这些生化机制,这些新颖的“原子药物”会激发其宝贵的生化特性并确定新的医学用途。
更新日期:2018-08-13
中文翻译:

解码稀有气体的丰富生物特性:我们如何预测稀有气体与多种蛋白质的结合?
具有化学惰性的稀有气体显示出令人惊讶的丰富的有用生物学特性光谱。关于这些作用背后的分子机制知之甚少。在稀有气体上进行大量药理实验以鉴定活性显然是不可行的。稀有气体与蛋白质结合的计算研究可以解决这种信息匮乏的问题,并提供对作用机理的深入了解。我们使用了定制的计算网格计算来预测在稀有气体与各种蛋白质相互作用中能量极小值的位置。通过量化模拟如何很好地预测包含399个Xe和Kr原子的131种不同蛋白质X射线结构中的结合位置来验证该方法的有效性。我们发现稀有气体的计算结合位置与实验结合位置之间具有极好的一致性。所有晶体氙原子的94%在预测结合位点的1 Xe van der Waals(vdW)直径之内,而97%位于2 vdW直径之内。100%的结晶k原子在预测结合位点的1 Kr vdW直径内。我们展示了蛋白质数据库中所有≈60000个独特结构的大规模计算筛选的可行性。这将阐明生物化学机制,通过这些生物化学机制,这些新颖的“原子药物”会激发其宝贵的生物化学特性并确定新的医学用途。100%的结晶k原子在预测结合位点的1 Kr vdW直径内。我们展示了蛋白质数据库中所有≈60000个独特结构的大规模计算筛选的可行性。这将阐明生化机制,通过这些生化机制,这些新颖的“原子药物”会激发其宝贵的生化特性并确定新的医学用途。100%的结晶k原子在预测结合位点的1 Kr vdW直径内。我们展示了蛋白质数据库中所有≈60000个独特结构的大规模计算筛选的可行性。这将阐明生化机制,通过这些生化机制,这些新颖的“原子药物”会激发其宝贵的生化特性并确定新的医学用途。


























 京公网安备 11010802027423号
京公网安备 11010802027423号