当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular Determinants of Substrate Specificity in Human Insulin-Degrading Enzyme
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1021/acs.biochem.8b00474 Lazaros Stefanidis 1 , Nicholas D. Fusco 1 , Samantha E. Cooper 2 , Jillian E. Smith-Carpenter 2 , Benjamin J. Alper 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1021/acs.biochem.8b00474 Lazaros Stefanidis 1 , Nicholas D. Fusco 1 , Samantha E. Cooper 2 , Jillian E. Smith-Carpenter 2 , Benjamin J. Alper 1
Affiliation
|
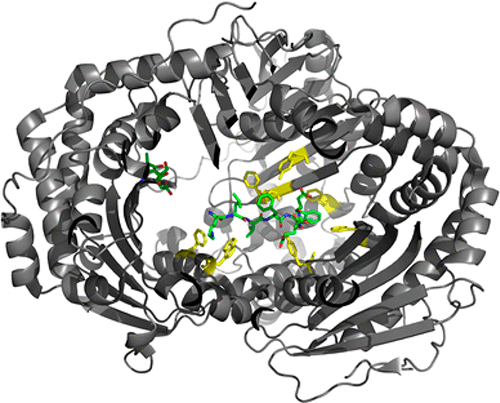
Insulin-degrading enzyme (IDE) is a 110 kDa chambered zinc metalloendopeptidase that degrades insulin, amyloid β, and other intermediate-sized aggregation prone peptides that adopt β-structures. Structural studies of IDE in complex with multiple physiological substrates have suggested a role for hydrophobic and aromatic residues of the IDE active site in substrate binding and catalysis. Here, we examine functional requirements for conserved hydrophobic and aromatic IDE active site residues that are positioned within 4.5 Å of IDE-bound insulin B chain and amyloid β peptides in the reported crystal structures for the respective enzyme–substrate complexes. Charge, size, hydrophobicity, aromaticity, and other functional group requirements for substrate binding IDE active site residues were examined through mutational analysis of the recombinant human enzyme and enzyme kinetic studies conducted using native and fluorogenic derivatives of human insulin and amyloid β peptides. A functional requirement for IDE active site residues F115, A140, F141, Y150, W199, F202, F820, and Y831 was established, and specific contributions of residue charge, size, and hydrophobicity to substrate binding, specificity, and proteolysis were demonstrated. IDE mutant alleles that exhibited enhanced or diminished proteolytic activity toward insulin or amyloid β peptides and derivative substrates were identified.
中文翻译:

人胰岛素降解酶中底物特异性的分子决定因素
胰岛素降解酶(IDE)是一种110 kDa的带腔室的锌金属内肽酶,可降解胰岛素,淀粉样蛋白β和其他采用β结构的中等大小的易于凝集的肽。对具有多种生理底物的IDE的结构研究表明,IDE活性位点的疏水和芳香残基在底物结合和催化中起着作用。在这里,我们检查了保守的疏水和芳香族IDE活性位点残基的功能要求,这些残基位于报告的相应酶-底物复合物晶体结构中IDE结合的胰岛素B链和淀粉样β肽4.5Å之内。电荷,大小,疏水性,芳香性,通过重组人酶的突变分析以及使用人胰岛素和淀粉样β肽的天然和荧光衍生物进行的酶动力学研究,检查了底物结合IDE活性位点残基的其他功能基团要求和酶动力学研究。建立了IDE活性位点残基F115,A140,F141,Y150,W199,F202,F820和Y831的功能要求,并证明了残基电荷,大小和疏水性对底物结合,特异性和蛋白水解的特定作用。鉴定出对胰岛素或淀粉样蛋白β肽和衍生物底物表现出增强或减弱的蛋白水解活性的IDE突变等位基因。建立了IDE活性位点残基F115,A140,F141,Y150,W199,F202,F820和Y831的功能要求,并证明了残基电荷,大小和疏水性对底物结合,特异性和蛋白水解的特定作用。鉴定出对胰岛素或淀粉样蛋白β肽和衍生物底物表现出增强或减弱的蛋白水解活性的IDE突变等位基因。建立了IDE活性位点残基F115,A140,F141,Y150,W199,F202,F820和Y831的功能要求,并证明了残基电荷,大小和疏水性对底物结合,特异性和蛋白水解的特定作用。鉴定出对胰岛素或淀粉样蛋白β肽和衍生物底物表现出增强或减弱的蛋白水解活性的IDE突变等位基因。
更新日期:2018-07-13
中文翻译:

人胰岛素降解酶中底物特异性的分子决定因素
胰岛素降解酶(IDE)是一种110 kDa的带腔室的锌金属内肽酶,可降解胰岛素,淀粉样蛋白β和其他采用β结构的中等大小的易于凝集的肽。对具有多种生理底物的IDE的结构研究表明,IDE活性位点的疏水和芳香残基在底物结合和催化中起着作用。在这里,我们检查了保守的疏水和芳香族IDE活性位点残基的功能要求,这些残基位于报告的相应酶-底物复合物晶体结构中IDE结合的胰岛素B链和淀粉样β肽4.5Å之内。电荷,大小,疏水性,芳香性,通过重组人酶的突变分析以及使用人胰岛素和淀粉样β肽的天然和荧光衍生物进行的酶动力学研究,检查了底物结合IDE活性位点残基的其他功能基团要求和酶动力学研究。建立了IDE活性位点残基F115,A140,F141,Y150,W199,F202,F820和Y831的功能要求,并证明了残基电荷,大小和疏水性对底物结合,特异性和蛋白水解的特定作用。鉴定出对胰岛素或淀粉样蛋白β肽和衍生物底物表现出增强或减弱的蛋白水解活性的IDE突变等位基因。建立了IDE活性位点残基F115,A140,F141,Y150,W199,F202,F820和Y831的功能要求,并证明了残基电荷,大小和疏水性对底物结合,特异性和蛋白水解的特定作用。鉴定出对胰岛素或淀粉样蛋白β肽和衍生物底物表现出增强或减弱的蛋白水解活性的IDE突变等位基因。建立了IDE活性位点残基F115,A140,F141,Y150,W199,F202,F820和Y831的功能要求,并证明了残基电荷,大小和疏水性对底物结合,特异性和蛋白水解的特定作用。鉴定出对胰岛素或淀粉样蛋白β肽和衍生物底物表现出增强或减弱的蛋白水解活性的IDE突变等位基因。



























 京公网安备 11010802027423号
京公网安备 11010802027423号