Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coordination‐Responsive Longitudinal Relaxation Tuning as a Versatile MRI Sensing Protocol for Malignancy Targets
Advanced Science ( IF 15.1 ) Pub Date : 2018-07-10 , DOI: 10.1002/advs.201800021 Kun Zhang 1, 2 , Yu Cheng 3 , Weiwei Ren 1 , Liping Sun 1 , Chang Liu 1 , Dan Wang 1 , Lehang Guo 1 , Huixiong Xu 1 , Yongxiang Zhao 2
Advanced Science ( IF 15.1 ) Pub Date : 2018-07-10 , DOI: 10.1002/advs.201800021 Kun Zhang 1, 2 , Yu Cheng 3 , Weiwei Ren 1 , Liping Sun 1 , Chang Liu 1 , Dan Wang 1 , Lehang Guo 1 , Huixiong Xu 1 , Yongxiang Zhao 2
Affiliation
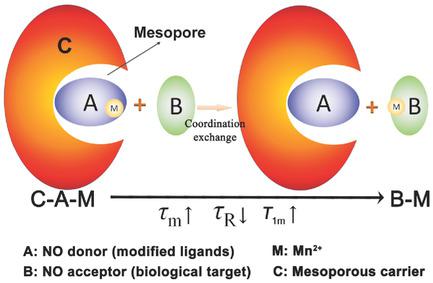
|
Biomarkers (e.g., acidity, H2O2, hypoxia, and specific molecules) as one primary component of tumor microenvironments are closely associated with occurrence, invasion, and metastasis of malignancy, thus can act as biological targets. However, their monitoring remains a challenging task. Herein, a coordination‐dependent longitudinal relaxation tuning (CLRT) that occurs between a Mn2+ “donor” and a Mn2+ “acceptor” is established to enable biological target sensing. Relying on the differences of coordination ability and spatial structure between donors and acceptors, the biological targets as Mn2+ acceptor can take Mn2+ away from the donors (i.e., modified ligands) in nanoscale probes, which consequently varies T1‐weighted (T1W) magnetic resonance imaging (MRI) signal. The coordination ability and spatial structure of the modified Mn2+ “donor” and the pore diameter of donor carrier are demonstrated to determine the feasibility, specificity, and generality of CLRT. With CLRT, this MRI‐based ruler is demonstrated for the successful specific detection of biological targets (i.e., hyaluronic acid and glutathione) of malignancy, and its potential in quantitative measurement of hyaluronic acid is further demonstrated. CLRT can serve as a novel and general sensing principle to augment the exploration of a wide range of biological systems.
中文翻译:

协调响应纵向弛豫调谐作为恶性肿瘤靶标的多功能 MRI 传感协议
生物标志物(如酸度、H 2 O 2、缺氧和特定分子)作为肿瘤微环境的主要组成部分,与恶性肿瘤的发生、侵袭和转移密切相关,因此可以作为生物靶点。然而,他们的监测仍然是一项具有挑战性的任务。在此,建立了Mn 2+ “供体”和 Mn 2+ “受体”之间发生的协调依赖性纵向弛豫调谐(CLRT),以实现生物目标传感。依靠供体和受体之间配位能力和空间结构的差异,作为Mn 2+受体的生物靶标可以在纳米级探针中将Mn 2+从供体(即修饰配体)上夺走,从而改变T1加权(T1W) ) 磁共振成像 (MRI) 信号。证明了修饰的Mn 2+ “供体”的配位能力和空间结构以及供体载体的孔径,以确定CLRT的可行性、特异性和通用性。通过 CLRT,这种基于 MRI 的标尺被证明可以成功特异性检测恶性肿瘤的生物靶标(即透明质酸和谷胱甘肽),并进一步证明其在透明质酸定量测量方面的潜力。CLRT 可以作为一种新颖且通用的传感原理来增强对各种生物系统的探索。
更新日期:2018-07-10
中文翻译:

协调响应纵向弛豫调谐作为恶性肿瘤靶标的多功能 MRI 传感协议
生物标志物(如酸度、H 2 O 2、缺氧和特定分子)作为肿瘤微环境的主要组成部分,与恶性肿瘤的发生、侵袭和转移密切相关,因此可以作为生物靶点。然而,他们的监测仍然是一项具有挑战性的任务。在此,建立了Mn 2+ “供体”和 Mn 2+ “受体”之间发生的协调依赖性纵向弛豫调谐(CLRT),以实现生物目标传感。依靠供体和受体之间配位能力和空间结构的差异,作为Mn 2+受体的生物靶标可以在纳米级探针中将Mn 2+从供体(即修饰配体)上夺走,从而改变T1加权(T1W) ) 磁共振成像 (MRI) 信号。证明了修饰的Mn 2+ “供体”的配位能力和空间结构以及供体载体的孔径,以确定CLRT的可行性、特异性和通用性。通过 CLRT,这种基于 MRI 的标尺被证明可以成功特异性检测恶性肿瘤的生物靶标(即透明质酸和谷胱甘肽),并进一步证明其在透明质酸定量测量方面的潜力。CLRT 可以作为一种新颖且通用的传感原理来增强对各种生物系统的探索。


























 京公网安备 11010802027423号
京公网安备 11010802027423号