当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aniracetam Solubility in Pure and Binary Solvents: Effect of Molecular Interaction and Analysis of Crystallized Products
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-07-10 , DOI: 10.1021/acs.jced.8b00023 Danfeng Shao 1 , Zehui Yang 1 , Guoquan Zhou 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-07-10 , DOI: 10.1021/acs.jced.8b00023 Danfeng Shao 1 , Zehui Yang 1 , Guoquan Zhou 1
Affiliation
|
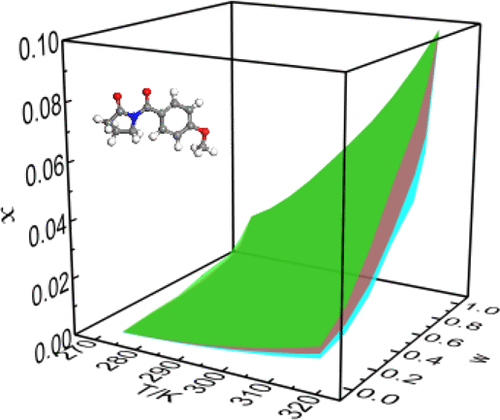
The aim of this work is to study the solubility of aniracetam and discuss the molecular interaction between solute and solvent in the dissolution process. This work would be important to optimize the crystallization and extraction process of aniracetam. The equilibrium data of aniracetam in seven pure solvents including methanol, ethanol, n-propanol, isopropyl alcohol, acetone, toluene, ethyl acetate, and three binary mixtures were measured by using the isothermal saturation method from T = 273.15 to 318.15 K. The solubility of aniracetam in pure and mixed solvents increased with the increasing temperature. At a given temperature, the solubility of aniracetam in (acetone + alcohols) increased with increasing mass fraction of acetone. Moreover, it was greater in (acetone + methanol) than other mixed systems. The maximum solubility of aniracetam in pure solvents was obtained in acetone, and the order they follow from small to large is (n-propanol < isopropyl alcohol < ethanol < methanol < toluene < ethyl acetate < acetone). Two pure solvent models (modified Apelblat equation, λh equation) and two cosolvent models (CNIBS/R-K model and Jouyban–Acree model) were applied to analyze the obtained solubility data. The correlation showed the experiment data is very close to the calculated values and exhibit low values of RAD and RMSD. Infrared spectra recorded between crystallized products and raw material in the pure dry KBr matrix in pairs and Pearson correlation coefficient were used to quantify the degree of similarity. Furthermore, powder X-ray diffraction (PXRD) was applied before and after the experiments to analyze the crystal form of aniracetam studied in this work.
中文翻译:

阿尼西坦在纯溶剂和二元溶剂中的溶解度:分子相互作用的影响和结晶产物的分析
这项工作的目的是研究阿尼西坦的溶解度,并讨论溶出过程中溶质与溶剂之间的分子相互作用。这项工作对于优化阿尼西坦的结晶和提取工艺将非常重要。阿尼西坦的七个纯溶剂的平衡数据,包括甲醇,乙醇,Ñ丙醇,异丙醇,丙酮,甲苯,乙酸乙酯,和三个二元混合物通过使用等温饱和方法从测得的Ť= 273.15至318.15K。阿尼西坦在纯溶剂和混合溶剂中的溶解度随温度的升高而增加。在给定温度下,阿尼西坦在(丙酮+醇)中的溶解度随丙酮质量分数的增加而增加。而且,它在(丙酮+甲醇)中的比其他混合体系要大。在丙酮中获得了阿尼西坦在纯溶剂中的最大溶解度,从小到大依次为(正丙醇<异丙醇<乙醇<甲醇<甲苯<乙酸乙酯<乙酸乙酯<丙酮)。两种纯溶剂模型(修正的Apelblat方程,λh方程)和两个共溶剂模型(CNIBS / RK模型和Jouyban–Acree模型)用于分析获得的溶解度数据。相关性表明实验数据与计算值非常接近,并且RAD和RMSD的值较低。使用成对的纯干KBr基质中结晶产物和原料之间记录的红外光谱和Pearson相关系数来量化相似程度。此外,在实验前后采用粉末X射线衍射(PXRD)分析在这项工作中研究的阿尼西坦的晶体形式。
更新日期:2018-07-12
中文翻译:

阿尼西坦在纯溶剂和二元溶剂中的溶解度:分子相互作用的影响和结晶产物的分析
这项工作的目的是研究阿尼西坦的溶解度,并讨论溶出过程中溶质与溶剂之间的分子相互作用。这项工作对于优化阿尼西坦的结晶和提取工艺将非常重要。阿尼西坦的七个纯溶剂的平衡数据,包括甲醇,乙醇,Ñ丙醇,异丙醇,丙酮,甲苯,乙酸乙酯,和三个二元混合物通过使用等温饱和方法从测得的Ť= 273.15至318.15K。阿尼西坦在纯溶剂和混合溶剂中的溶解度随温度的升高而增加。在给定温度下,阿尼西坦在(丙酮+醇)中的溶解度随丙酮质量分数的增加而增加。而且,它在(丙酮+甲醇)中的比其他混合体系要大。在丙酮中获得了阿尼西坦在纯溶剂中的最大溶解度,从小到大依次为(正丙醇<异丙醇<乙醇<甲醇<甲苯<乙酸乙酯<乙酸乙酯<丙酮)。两种纯溶剂模型(修正的Apelblat方程,λh方程)和两个共溶剂模型(CNIBS / RK模型和Jouyban–Acree模型)用于分析获得的溶解度数据。相关性表明实验数据与计算值非常接近,并且RAD和RMSD的值较低。使用成对的纯干KBr基质中结晶产物和原料之间记录的红外光谱和Pearson相关系数来量化相似程度。此外,在实验前后采用粉末X射线衍射(PXRD)分析在这项工作中研究的阿尼西坦的晶体形式。


























 京公网安备 11010802027423号
京公网安备 11010802027423号