当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of α-oxygenated β,γ-unsaturated ketones by a catalytic rearrangement strategy†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1039/c8ob01559g Luisa Lempenauer 1, 2, 3, 4, 5 , Aline Soupart 1, 2, 3, 4, 5 , Elisabet Duñach 1, 2, 3, 4, 5 , Gilles Lemière 5, 6, 7
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1039/c8ob01559g Luisa Lempenauer 1, 2, 3, 4, 5 , Aline Soupart 1, 2, 3, 4, 5 , Elisabet Duñach 1, 2, 3, 4, 5 , Gilles Lemière 5, 6, 7
Affiliation
|
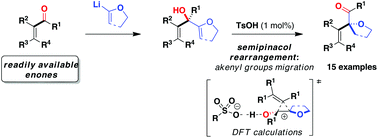
A straightforward two-step entry to α-oxgenated β,γ-unsaturated ketones from readily available α,β-unsaturated ketones is disclosed. It was found that bis(allylic) alcohols undergo a skeletal rearrangement in the presence of 1 mol% of cheap and non-corrosive p-toluenesulfonic acid. Computational studies were conducted to support the mechanism and to rationalise the influence of the catalyst acidity on the product selectivity.
中文翻译:

催化重排策略合成α-氧化的β,γ-不饱和酮†
公开了从容易获得的α,β-不饱和酮直接进入α-加氧的β,γ-不饱和酮的两步法。发现双(烯丙基)醇在1mol%的廉价且无腐蚀性的对甲苯磺酸的存在下发生骨架重排。进行了计算研究以支持该机理并合理化催化剂酸度对产物选择性的影响。
更新日期:2018-07-11
中文翻译:

催化重排策略合成α-氧化的β,γ-不饱和酮†
公开了从容易获得的α,β-不饱和酮直接进入α-加氧的β,γ-不饱和酮的两步法。发现双(烯丙基)醇在1mol%的廉价且无腐蚀性的对甲苯磺酸的存在下发生骨架重排。进行了计算研究以支持该机理并合理化催化剂酸度对产物选择性的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号