当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly efficient synthesis of benzodioxins with a 2-site quaternary carbon structure by secondary amine-catalyzed dual Michael cascade reactions†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-07-10 00:00:00 , DOI: 10.1039/c8ob01029c Xuefeng He 1, 2, 3, 4, 5 , Yongsu Li 1, 2, 3, 4, 5 , Meng Wang 1, 2, 3, 4, 5 , Hui-Xuan Chen 1, 2, 3, 4, 5 , Bin Chen 1, 2, 3, 4, 5 , Hao Liang 1, 2, 3, 4, 5 , Yaqi Zhang 1, 2, 3, 4, 5 , Jiyan Pang 1, 2, 3, 4, 5 , Liqin Qiu 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-07-10 00:00:00 , DOI: 10.1039/c8ob01029c Xuefeng He 1, 2, 3, 4, 5 , Yongsu Li 1, 2, 3, 4, 5 , Meng Wang 1, 2, 3, 4, 5 , Hui-Xuan Chen 1, 2, 3, 4, 5 , Bin Chen 1, 2, 3, 4, 5 , Hao Liang 1, 2, 3, 4, 5 , Yaqi Zhang 1, 2, 3, 4, 5 , Jiyan Pang 1, 2, 3, 4, 5 , Liqin Qiu 1, 2, 3, 4, 5
Affiliation
|
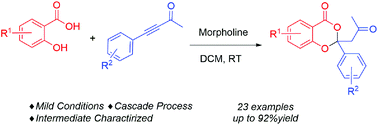
Salicylic acids and substituted ynones were employed as substrates to afford a class of valuable 4H-benzo[d][1,3]dioxin-4-ones with a 2-site quaternary carbon structure in up to 92% yield by secondary amine-catalyzed dual Michael cascade reactions under mild reaction conditions. The α,β-unsaturated ketone as the key intermediate in the cascade process was successfully separated and characterized. As a result, a new reaction route for ynone species is demonstrated, which is totally different from the existing allenamine activation model.
中文翻译:

通过仲胺催化的双Michael级联反应高效合成具有2位季碳结构的苯并二恶英†
水杨酸和取代的炔酮被用作底物,以提供一类有价值的4 H-苯并[ d ] [1,3]二恶英-4-酮,其具有2位季碳结构,仲胺-收率最高可达92%。在温和的反应条件下催化双重Michael级联反应。成功地分离和表征了α,β-不饱和酮作为级联过程中的关键中间体。结果,证明了一种用于炔酮的新反应路线,这与现有的艾伦胺活化模型完全不同。
更新日期:2018-07-10
中文翻译:

通过仲胺催化的双Michael级联反应高效合成具有2位季碳结构的苯并二恶英†
水杨酸和取代的炔酮被用作底物,以提供一类有价值的4 H-苯并[ d ] [1,3]二恶英-4-酮,其具有2位季碳结构,仲胺-收率最高可达92%。在温和的反应条件下催化双重Michael级联反应。成功地分离和表征了α,β-不饱和酮作为级联过程中的关键中间体。结果,证明了一种用于炔酮的新反应路线,这与现有的艾伦胺活化模型完全不同。



























 京公网安备 11010802027423号
京公网安备 11010802027423号