当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Base controlled diverse reactivity of allyl cyanide for synthesis of multi-substituted benzenes†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-07-10 00:00:00 , DOI: 10.1039/c8ob01270a Pratik Yadav 1, 2, 3, 4 , Ranjay Shaw 1, 2, 3, 4 , Amr Elagamy 1, 2, 3, 4 , Abhinav Kumar 1, 4, 5, 6 , Ramendra Pratap 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-07-10 00:00:00 , DOI: 10.1039/c8ob01270a Pratik Yadav 1, 2, 3, 4 , Ranjay Shaw 1, 2, 3, 4 , Amr Elagamy 1, 2, 3, 4 , Abhinav Kumar 1, 4, 5, 6 , Ramendra Pratap 1, 2, 3, 4
Affiliation
|
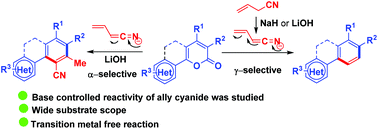
A base controlled regioselective 1,6-cyanoallylation of suitably functionalized 2H-pyran-2-ones has been demonstrated for the synthesis of various multi-substituted benzenes through a tandem process. We observed that lithium hydroxide provides a major product from α-attack and a minor product from γ-attack of allyl cyanide, while the use of sodium hydride as a base exclusively provides the product by γ-attack of allyl cyanide. We have also performed NMR experiments to understand the mechanistic pathway. The structure of the compound was confirmed by single crystal X-ray analysis.
中文翻译:

碱控制的烯丙基氰化物的多种反应活性,用于合成多取代苯†
已经证明了适当官能化的2 H-吡喃-2-酮的碱控制的区域选择性1,6-氰基烯丙基化用于通过串联方法合成各种多取代的苯。我们观察到,氢氧化锂提供的主要产物是α-攻击,而次要的产物是γ-烯丙基氰,而氢化钠作为碱只能提供γ-攻击的烯丙基氰。我们还进行了NMR实验,以了解机理。该化合物的结构通过单晶X射线分析确认。
更新日期:2018-07-10
中文翻译:

碱控制的烯丙基氰化物的多种反应活性,用于合成多取代苯†
已经证明了适当官能化的2 H-吡喃-2-酮的碱控制的区域选择性1,6-氰基烯丙基化用于通过串联方法合成各种多取代的苯。我们观察到,氢氧化锂提供的主要产物是α-攻击,而次要的产物是γ-烯丙基氰,而氢化钠作为碱只能提供γ-攻击的烯丙基氰。我们还进行了NMR实验,以了解机理。该化合物的结构通过单晶X射线分析确认。

























 京公网安备 11010802027423号
京公网安备 11010802027423号