当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Absolute Estimates of PdII(η2-Arene) C–H Acidity
Organometallics ( IF 2.8 ) Pub Date : 2018-07-10 , DOI: 10.1021/acs.organomet.8b00348 William E. Christman 1 , Travis J. Morrow 1 , Navamoney Arulsamy 1 , Elliott B. Hulley 1
Organometallics ( IF 2.8 ) Pub Date : 2018-07-10 , DOI: 10.1021/acs.organomet.8b00348 William E. Christman 1 , Travis J. Morrow 1 , Navamoney Arulsamy 1 , Elliott B. Hulley 1
Affiliation
|
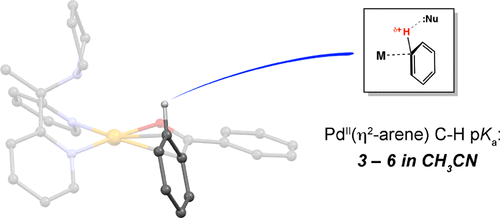
Thermodynamic acidity is one of the most widely used quantities for characterizing proton transfer reactions. Measurement of these values for catalytically relevant species can be challenging, often requiring direct observation of equilibria. The C–H bonds of aromatic substrates are proposed to become substantially polarized during electrophilic activation, but quantifying the absolute acidity of the intermediate M(η2-arene) complexes is highly challenging. Using a system that intercepts nascent protons at electrophilic PdII arene complexes, a combined experimental and computational study has demonstrated these C–H bonds to be far more acidic (pKaCH3CN = 3–6) than many “nonbasic” substrates and additives that are present in electrophilic C–H activation catalysis, and the catalytic roles of these species may need to be reassessed.
中文翻译:

钯的绝对估计II(η 2 -Arene)C-H酸度
热力学酸度是表征质子转移反应最广泛使用的量之一。对于催化相关物种,这些值的测量可能具有挑战性,通常需要直接观察平衡。芳族底物的C-H键被提出成为亲电活化期间显着偏振的,但量化中间体M的绝对酸度(η 2 -arene)配合物是高度挑战。通过使用在亲电的Pd II芳烃络合物上拦截新生质子的系统,结合的实验和计算研究表明,这些C–H键的酸性更高(p K a CH 3 CN = 3–6),而不是亲电子C–H活化催化中存在的许多“非碱性”底物和添加剂,可能需要重新评估这些物质的催化作用。
更新日期:2018-07-12
中文翻译:

钯的绝对估计II(η 2 -Arene)C-H酸度
热力学酸度是表征质子转移反应最广泛使用的量之一。对于催化相关物种,这些值的测量可能具有挑战性,通常需要直接观察平衡。芳族底物的C-H键被提出成为亲电活化期间显着偏振的,但量化中间体M的绝对酸度(η 2 -arene)配合物是高度挑战。通过使用在亲电的Pd II芳烃络合物上拦截新生质子的系统,结合的实验和计算研究表明,这些C–H键的酸性更高(p K a CH 3 CN = 3–6),而不是亲电子C–H活化催化中存在的许多“非碱性”底物和添加剂,可能需要重新评估这些物质的催化作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号