Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
PtdIns(4,5)P2 stabilizes active states of GPCRs and enhances selectivity of G-protein coupling
Nature ( IF 64.8 ) Pub Date : 2018-07-01 , DOI: 10.1038/s41586-018-0325-6 Hsin-Yung Yen 1, 2 , Kin Kuan Hoi 1 , Idlir Liko 1, 2 , George Hedger 3 , Michael R Horrell 3 , Wanling Song 3 , Di Wu 1 , Philipp Heine 4 , Tony Warne 5 , Yang Lee 5 , Byron Carpenter 5, 6 , Andreas Plückthun 4 , Christopher G Tate 5 , Mark S P Sansom 3 , Carol V Robinson 1
Nature ( IF 64.8 ) Pub Date : 2018-07-01 , DOI: 10.1038/s41586-018-0325-6 Hsin-Yung Yen 1, 2 , Kin Kuan Hoi 1 , Idlir Liko 1, 2 , George Hedger 3 , Michael R Horrell 3 , Wanling Song 3 , Di Wu 1 , Philipp Heine 4 , Tony Warne 5 , Yang Lee 5 , Byron Carpenter 5, 6 , Andreas Plückthun 4 , Christopher G Tate 5 , Mark S P Sansom 3 , Carol V Robinson 1
Affiliation
|
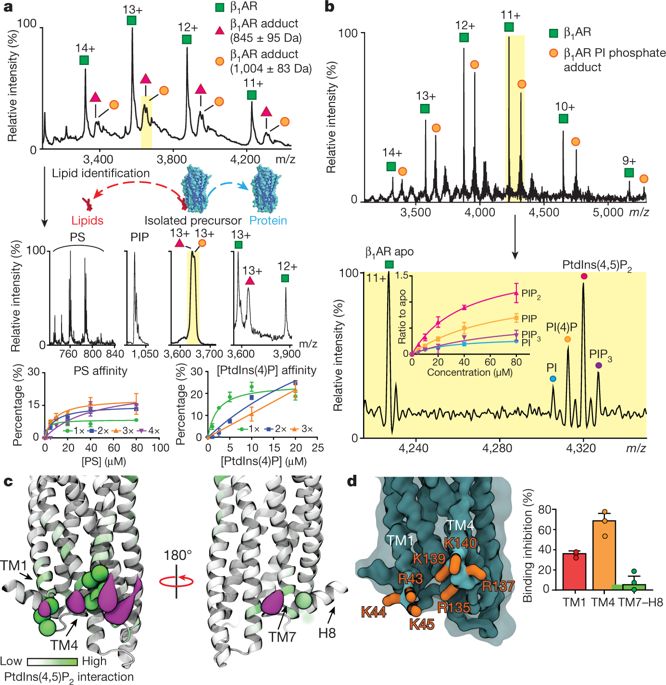
G-protein-coupled receptors (GPCRs) are involved in many physiological processes and are therefore key drug targets1. Although detailed structural information is available for GPCRs, the effects of lipids on the receptors, and on downstream coupling of GPCRs to G proteins are largely unknown. Here we use native mass spectrometry to identify endogenous lipids bound to three class A GPCRs. We observed preferential binding of phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) over related lipids and confirm that the intracellular surface of the receptors contain hotspots for PtdIns(4,5)P2 binding. Endogenous lipids were also observed bound directly to the trimeric Gαsβγ protein complex of the adenosine A2A receptor (A2AR) in the gas phase. Using engineered Gα subunits (mini-Gαs, mini-Gαi and mini-Gα12)2, we demonstrate that the complex of mini-Gαs with the β1 adrenergic receptor (β1AR) is stabilized by the binding of two PtdIns(4,5)P2 molecules. By contrast, PtdIns(4,5)P2 does not stabilize coupling between β1AR and other Gα subunits (mini-Gαi or mini-Gα12) or a high-affinity nanobody. Other endogenous lipids that bind to these receptors have no effect on coupling, highlighting the specificity of PtdIns(4,5)P2. Calculations of potential of mean force and increased GTP turnover by the activated neurotensin receptor when coupled to trimeric Gαiβγ complex in the presence of PtdIns(4,5)P2 provide further evidence for a specific effect of PtdIns(4,5)P2 on coupling. We identify key residues on cognate Gα subunits through which PtdIns(4,5)P2 forms bridging interactions with basic residues on class A GPCRs. These modulating effects of lipids on receptors suggest consequences for understanding function, G-protein selectivity and drug targeting of class A GPCRs.Mass spectrometry-based assays are used to reveal specificity and structural determinants of lipid binding to class A G-protein-coupled receptors, and the effects of specific lipids on receptor coupling to G proteins.
中文翻译:

PtdIns(4,5)P2 稳定 GPCR 的活性状态并增强 G 蛋白偶联的选择性
G 蛋白偶联受体 (GPCR) 参与许多生理过程,因此是关键的药物靶点1。尽管可以获得 GPCR 的详细结构信息,但脂质对受体以及 GPCR 与 G 蛋白下游偶联的影响在很大程度上尚不清楚。在这里,我们使用天然质谱法来鉴定与三种 A 类 GPCR 结合的内源脂质。我们观察到磷脂酰肌醇-4,5-二磷酸 (PtdIns(4,5)P2) 比相关脂质优先结合,并证实受体的细胞内表面包含 PtdIns(4,5)P2 结合的热点。还观察到内源性脂质在气相中直接与腺苷 A2A 受体 (A2AR) 的三聚体 Gαsβγ 蛋白复合物结合。使用工程化的 Gα 亚基(mini-Gαs、mini-Gαi 和 mini-Gα12)2,我们证明 mini-Gαs 与 β1 肾上腺素能受体 (β1AR) 的复合物通过两个 PtdIns(4,5)P2 的结合而稳定分子。相比之下,PtdIns(4,5)P2 不能稳定 β1AR 与其他 Gα 亚基(mini-Gαi 或 mini-Gα12)或高亲和力纳米抗体之间的偶联。与这些受体结合的其他内源脂质对偶联没有影响,突出了 PtdIns(4,5)P2 的特异性。当在 PtdIns(4,5)P2 存在的情况下与三聚体 Gαiβγ 复合物偶联时,激活的神经降压素受体的平均力潜力和 GTP 周转增加的计算为 PtdIns(4,5)P2 对偶联的特定作用提供了进一步的证据。我们确定了同源 Gα 亚基上的关键残基,PtdIns(4,5)P2 通过这些残基与 A 类 GPCR 上的基本残基形成桥接相互作用。脂质对受体的这些调节作用表明对理解 A 类 GPCR 的功能、G 蛋白选择性和药物靶向的影响。基于质谱的测定用于揭示脂质与 A 类 G 蛋白偶联受体结合的特异性和结构决定因素,以及特定脂质对 G 蛋白受体偶联的影响。
更新日期:2018-07-01
中文翻译:

PtdIns(4,5)P2 稳定 GPCR 的活性状态并增强 G 蛋白偶联的选择性
G 蛋白偶联受体 (GPCR) 参与许多生理过程,因此是关键的药物靶点1。尽管可以获得 GPCR 的详细结构信息,但脂质对受体以及 GPCR 与 G 蛋白下游偶联的影响在很大程度上尚不清楚。在这里,我们使用天然质谱法来鉴定与三种 A 类 GPCR 结合的内源脂质。我们观察到磷脂酰肌醇-4,5-二磷酸 (PtdIns(4,5)P2) 比相关脂质优先结合,并证实受体的细胞内表面包含 PtdIns(4,5)P2 结合的热点。还观察到内源性脂质在气相中直接与腺苷 A2A 受体 (A2AR) 的三聚体 Gαsβγ 蛋白复合物结合。使用工程化的 Gα 亚基(mini-Gαs、mini-Gαi 和 mini-Gα12)2,我们证明 mini-Gαs 与 β1 肾上腺素能受体 (β1AR) 的复合物通过两个 PtdIns(4,5)P2 的结合而稳定分子。相比之下,PtdIns(4,5)P2 不能稳定 β1AR 与其他 Gα 亚基(mini-Gαi 或 mini-Gα12)或高亲和力纳米抗体之间的偶联。与这些受体结合的其他内源脂质对偶联没有影响,突出了 PtdIns(4,5)P2 的特异性。当在 PtdIns(4,5)P2 存在的情况下与三聚体 Gαiβγ 复合物偶联时,激活的神经降压素受体的平均力潜力和 GTP 周转增加的计算为 PtdIns(4,5)P2 对偶联的特定作用提供了进一步的证据。我们确定了同源 Gα 亚基上的关键残基,PtdIns(4,5)P2 通过这些残基与 A 类 GPCR 上的基本残基形成桥接相互作用。脂质对受体的这些调节作用表明对理解 A 类 GPCR 的功能、G 蛋白选择性和药物靶向的影响。基于质谱的测定用于揭示脂质与 A 类 G 蛋白偶联受体结合的特异性和结构决定因素,以及特定脂质对 G 蛋白受体偶联的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号