Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reprogramming human T cell function and specificity with non-viral genome targeting
Nature ( IF 64.8 ) Pub Date : 2018-07-01 , DOI: 10.1038/s41586-018-0326-5 Theodore L. Roth , Cristina Puig-Saus , Ruby Yu , Eric Shifrut , Julia Carnevale , P. Jonathan Li , Joseph Hiatt , Justin Saco , Paige Krystofinski , Han Li , Victoria Tobin , David N. Nguyen , Michael R. Lee , Amy L. Putnam , Andrea L. Ferris , Jeff W. Chen , Jean-Nicolas Schickel , Laurence Pellerin , David Carmody , Gorka Alkorta-Aranburu , Daniela del Gaudio , Hiroyuki Matsumoto , Montse Morell , Ying Mao , Min Cho , Rolen M. Quadros , Channabasavaiah B. Gurumurthy , Baz Smith , Michael Haugwitz , Stephen H. Hughes , Jonathan S. Weissman , Kathrin Schumann , Jonathan H. Esensten , Andrew P. May , Alan Ashworth , Gary M. Kupfer , Siri Atma W. Greeley , Rosa Bacchetta , Eric Meffre , Maria Grazia Roncarolo , Neil Romberg , Kevan C. Herold , Antoni Ribas , Manuel D. Leonetti , Alexander Marson
Nature ( IF 64.8 ) Pub Date : 2018-07-01 , DOI: 10.1038/s41586-018-0326-5 Theodore L. Roth , Cristina Puig-Saus , Ruby Yu , Eric Shifrut , Julia Carnevale , P. Jonathan Li , Joseph Hiatt , Justin Saco , Paige Krystofinski , Han Li , Victoria Tobin , David N. Nguyen , Michael R. Lee , Amy L. Putnam , Andrea L. Ferris , Jeff W. Chen , Jean-Nicolas Schickel , Laurence Pellerin , David Carmody , Gorka Alkorta-Aranburu , Daniela del Gaudio , Hiroyuki Matsumoto , Montse Morell , Ying Mao , Min Cho , Rolen M. Quadros , Channabasavaiah B. Gurumurthy , Baz Smith , Michael Haugwitz , Stephen H. Hughes , Jonathan S. Weissman , Kathrin Schumann , Jonathan H. Esensten , Andrew P. May , Alan Ashworth , Gary M. Kupfer , Siri Atma W. Greeley , Rosa Bacchetta , Eric Meffre , Maria Grazia Roncarolo , Neil Romberg , Kevan C. Herold , Antoni Ribas , Manuel D. Leonetti , Alexander Marson
|
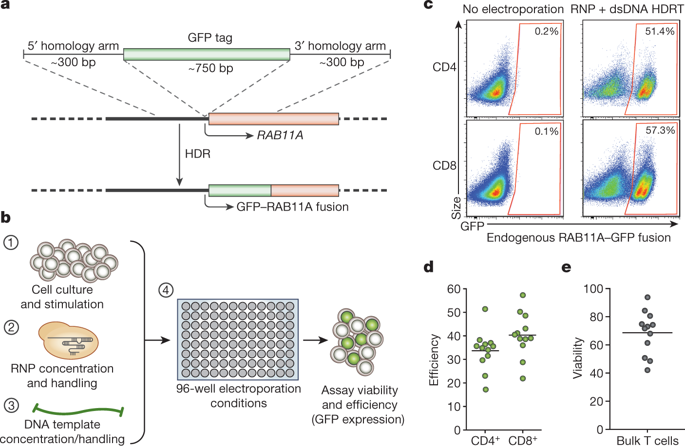
Decades of work have aimed to genetically reprogram T cells for therapeutic purposes1,2 using recombinant viral vectors, which do not target transgenes to specific genomic sites3,4. The need for viral vectors has slowed down research and clinical use as their manufacturing and testing is lengthy and expensive. Genome editing brought the promise of specific and efficient insertion of large transgenes into target cells using homology-directed repair5,6. Here we developed a CRISPR–Cas9 genome-targeting system that does not require viral vectors, allowing rapid and efficient insertion of large DNA sequences (greater than one kilobase) at specific sites in the genomes of primary human T cells, while preserving cell viability and function. This permits individual or multiplexed modification of endogenous genes. First, we applied this strategy to correct a pathogenic IL2RA mutation in cells from patients with monogenic autoimmune disease, and demonstrate improved signalling function. Second, we replaced the endogenous T cell receptor (TCR) locus with a new TCR that redirected T cells to a cancer antigen. The resulting TCR-engineered T cells specifically recognized tumour antigens and mounted productive anti-tumour cell responses in vitro and in vivo. Together, these studies provide preclinical evidence that non-viral genome targeting can enable rapid and flexible experimental manipulation and therapeutic engineering of primary human immune cells.A non-viral strategy to introduce large DNA sequences into T cells enables the correction of a pathogenic mutation that causes autoimmunity, and the replacement of an endogenous T-cell receptor with an engineered receptor that can recognize cancer antigens.
中文翻译:

用非病毒基因组靶向重编程人类 T 细胞功能和特异性
数十年的工作旨在使用重组病毒载体对 T 细胞进行基因重编程以用于治疗目的 1,2,这些载体不将转基因靶向特定基因组位点 3,4。对病毒载体的需求减缓了研究和临床使用,因为它们的制造和测试既漫长又昂贵。基因组编辑带来了使用同源定向修复将大型转基因特异性和有效插入靶细胞的希望5,6。在这里,我们开发了一种不需要病毒载体的 CRISPR-Cas9 基因组靶向系统,允许在原代人类 T 细胞基因组中的特定位点快速有效地插入大 DNA 序列(大于 1 千碱基),同时保持细胞活力和功能。这允许对内源基因进行单独或多重修饰。第一的,我们应用这种策略来纠正单基因自身免疫疾病患者细胞中的致病性 IL2RA 突变,并证明信号功能得到改善。其次,我们用新的 TCR 替换了内源性 T 细胞受体 (TCR) 基因座,该 TCR 将 T 细胞重定向到癌症抗原。由此产生的 TCR 工程 T 细胞特异性识别肿瘤抗原,并在体外和体内产生有效的抗肿瘤细胞反应。总之,这些研究提供了临床前证据,表明非病毒基因组靶向可以实现对原代人类免疫细胞的快速和灵活的实验操作和治疗工程。将大 DNA 序列引入 T 细胞的非病毒策略可以纠正致病突变导致自身免疫,
更新日期:2018-07-01
中文翻译:

用非病毒基因组靶向重编程人类 T 细胞功能和特异性
数十年的工作旨在使用重组病毒载体对 T 细胞进行基因重编程以用于治疗目的 1,2,这些载体不将转基因靶向特定基因组位点 3,4。对病毒载体的需求减缓了研究和临床使用,因为它们的制造和测试既漫长又昂贵。基因组编辑带来了使用同源定向修复将大型转基因特异性和有效插入靶细胞的希望5,6。在这里,我们开发了一种不需要病毒载体的 CRISPR-Cas9 基因组靶向系统,允许在原代人类 T 细胞基因组中的特定位点快速有效地插入大 DNA 序列(大于 1 千碱基),同时保持细胞活力和功能。这允许对内源基因进行单独或多重修饰。第一的,我们应用这种策略来纠正单基因自身免疫疾病患者细胞中的致病性 IL2RA 突变,并证明信号功能得到改善。其次,我们用新的 TCR 替换了内源性 T 细胞受体 (TCR) 基因座,该 TCR 将 T 细胞重定向到癌症抗原。由此产生的 TCR 工程 T 细胞特异性识别肿瘤抗原,并在体外和体内产生有效的抗肿瘤细胞反应。总之,这些研究提供了临床前证据,表明非病毒基因组靶向可以实现对原代人类免疫细胞的快速和灵活的实验操作和治疗工程。将大 DNA 序列引入 T 细胞的非病毒策略可以纠正致病突变导致自身免疫,



























 京公网安备 11010802027423号
京公网安备 11010802027423号