当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Visible‐Light‐Mediated Photoredox‐Catalyzed N‐Arylation of NH‐Sulfoximines with Electron‐Rich Arenes
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-08-02 , DOI: 10.1002/adsc.201800607 Alexander Wimmer 1 , Burkhard König 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-08-02 , DOI: 10.1002/adsc.201800607 Alexander Wimmer 1 , Burkhard König 1
Affiliation
|
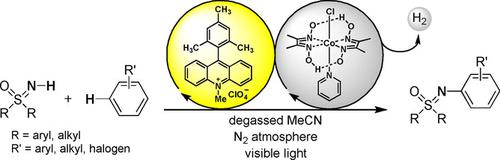
The direct C−H/N−H dehydrogenative cross‐coupling of NH‐sulfoximines with electron‐rich arenes was realized by oxidative visible‐light photoredox catalysis, applying 9‐mesityl‐10‐methylacridinium perchlorate as an organic photocatalyst. Sulfoximines display diverse desirable properties for medicinal chemistry and the pharmaceutical industry. However, their preparation is still challenging. Our reaction proceeds without sacrificial oxidant, at room temperature and is highly selective for the C−N bond forming reaction. The scope of the reaction includes mono‐ and multi‐alkylated and halogenated arenes, which are reacted with aromatic and aliphatic electron‐rich and electron‐poor NH‐sulfoximines, giving moderate to excellent yields of the N‐arylated sulfoximines. In addition, we successfully conducted the developed reaction on a gram scale (1.5 g). Mechanistic investigations show that both arene and NH‐sulfoximine interact with the excited‐state of the photocatalyst. We propose a radical‐based mechanism, where both the arene and the NH‐sulfoximine are photo‐oxidized to their respective radical intermediates. Radical‐radical cross‐coupling subsequently leads to the N‐arylated sulfoximine. Two electrons and two protons are released during the reaction and are subsequently converted into H2 by a proton‐reducing cobalt‐catalyst.
中文翻译:

可见光介导的光氧化还原催化NH-亚磺酰亚胺与富电子芳烃的N-芳基化
采用9-异三甲苯基-10-甲基吖啶鎓高氯酸盐作为有机光催化剂,通过氧化可见光光氧化还原催化实现了NH-亚磺酰亚胺与富电子芳烃的直接C-H/N-H脱氢交叉偶联。亚磺酰亚胺显示出药物化学和制药工业所需的多种特性。然而,他们的准备工作仍然充满挑战。我们的反应在室温下进行,无需牺牲氧化剂,并且对 CN 键形成反应具有高度选择性。该反应范围包括单烷基化和多烷基化以及卤代芳烃,它们与芳香族和脂肪族富电子和缺电子的NH-亚砜亚胺反应,得到N-芳基化亚砜亚胺的中等至优异的产率。此外,我们成功地在克级(1.5克)上进行了开发的反应。机理研究表明芳烃和NH-亚磺酰亚胺都与光催化剂的激发态相互作用。我们提出了一种基于自由基的机制,其中芳烃和NH-亚磺酰亚胺都被光氧化成各自的自由基中间体。自由基-自由基交叉偶联随后产生N-芳基化亚砜亚胺。反应过程中释放出两个电子和两个质子,随后通过质子还原钴催化剂转化为 H 2 。
更新日期:2018-08-02
中文翻译:

可见光介导的光氧化还原催化NH-亚磺酰亚胺与富电子芳烃的N-芳基化
采用9-异三甲苯基-10-甲基吖啶鎓高氯酸盐作为有机光催化剂,通过氧化可见光光氧化还原催化实现了NH-亚磺酰亚胺与富电子芳烃的直接C-H/N-H脱氢交叉偶联。亚磺酰亚胺显示出药物化学和制药工业所需的多种特性。然而,他们的准备工作仍然充满挑战。我们的反应在室温下进行,无需牺牲氧化剂,并且对 CN 键形成反应具有高度选择性。该反应范围包括单烷基化和多烷基化以及卤代芳烃,它们与芳香族和脂肪族富电子和缺电子的NH-亚砜亚胺反应,得到N-芳基化亚砜亚胺的中等至优异的产率。此外,我们成功地在克级(1.5克)上进行了开发的反应。机理研究表明芳烃和NH-亚磺酰亚胺都与光催化剂的激发态相互作用。我们提出了一种基于自由基的机制,其中芳烃和NH-亚磺酰亚胺都被光氧化成各自的自由基中间体。自由基-自由基交叉偶联随后产生N-芳基化亚砜亚胺。反应过程中释放出两个电子和两个质子,随后通过质子还原钴催化剂转化为 H 2 。


























 京公网安备 11010802027423号
京公网安备 11010802027423号