Joule ( IF 39.8 ) Pub Date : 2018-07-09 , DOI: 10.1016/j.joule.2018.06.015 Xiang Liu , Dongsheng Ren , Hungjen Hsu , Xuning Feng , Gui-Liang Xu , Minghao Zhuang , Han Gao , Languang Lu , Xuebing Han , Zhengyu Chu , Jianqiu Li , Xiangming He , Khalil Amine , Minggao Ouyang
|
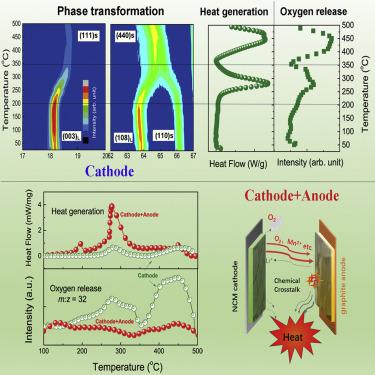
We demonstrate herein that not only internal short circuiting, but also chemical crossover, is the mechanism behind thermal runaway that can occur in lithium-ion batteries due to abuse conditions. In situ experiments showed that during thermal runaway, the cathode releases oxygen by a phase transition, and this oxygen is consumed by the lithiated anode. The released highly oxidative gas reacts with reductive LiCx with tremendous heat generation centered at 274.2°C with heat flow of 87.8 W g−1. To confirm the proposed mechanism, we froze a battery undergoing the thermal runaway process by liquid nitrogen and subjected it to detailed post-test analysis. Our results revealed the hidden thermal runaway mechanism of chemical crossover between the battery components without a severe internal short circuit. These findings provide an important insight into the rational design of automotive lithium-ion batteries as well as solid-state batteries.
中文翻译:

锂离子电池的热失控而无内部短路
我们在本文中证明,不仅内部短路而且化学交叉也是热失控背后的机制,该机制可能由于滥用条件而在锂离子电池中发生。原位实验表明,在热失控过程中,阴极通过相变释放出氧气,并且该氧气被锂化阳极消耗。释放出的高氧化性气体与还原性LiC x反应,产生大量热量,集中在274.2°C,热流为87.8 W g -1。为了确认提出的机制,我们用液氮冻结了经历热失控过程的电池,并对其进行了详细的测试后分析。我们的研究结果揭示了电池组件之间化学交叉的隐藏热失控机制,而没有严重的内部短路。这些发现为汽车锂离子电池以及固态电池的合理设计提供了重要的见识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号