当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reaction Mechanism with Thermodynamic Structural Screening for Electrochemical Hydrogen Evolution on Monolayer 1T′ Phase MoS2
Chemistry of Materials ( IF 8.6 ) Pub Date : 2018-07-08 00:00:00 , DOI: 10.1021/acs.chemmater.8b02236 Shiqi Chen 1 , Xiaobo Chen 1 , Guangjin Wang 2, 3 , Lu Liu 1 , Qiaoqiao He 1 , Xi-Bo Li 1 , Ni Cui 1
Chemistry of Materials ( IF 8.6 ) Pub Date : 2018-07-08 00:00:00 , DOI: 10.1021/acs.chemmater.8b02236 Shiqi Chen 1 , Xiaobo Chen 1 , Guangjin Wang 2, 3 , Lu Liu 1 , Qiaoqiao He 1 , Xi-Bo Li 1 , Ni Cui 1
Affiliation
|
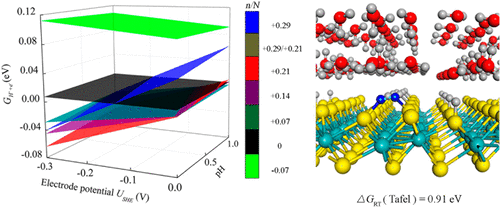
We report on a density functional theory (DFT) calculation to determine the reaction pathway and barrier of the hydrogen evolution reaction (HER) at the interface of monolayer 1T′ phase MoS2 and water. By screening the interfacial structures with the lowest chemical potential of protons and electrons, key structural characteristics that are important for prediction of reaction mechanism are identified. Under typical reaction conditions of HER, the catalyst surface features a high proton coverage of ca. 37% while the aqueous solution has a relatively low hydronium concentration of no more than ca. 1.8%. This contrast leads to proton desorption from the catalyst surface through a diffusion-assisted Tafel manner, rather than the Heyrovsky manner assumed previously. The result is supported by the agreement of the calculated reaction barrier and surface coverage with those of experimental estimate. In prediction of catalytic activity, hydrogen adsorption energies of reaction intermediates are widely used as the thermodynamic descriptor, while reaction barriers usually serve as kinetic parameters. We suggest that both thermodynamic and kinetic description toward HER should be performed on the premise that the lowest chemical potential of protons and electrons is obtained.
中文翻译:

热力学结构筛选反应机理的单层1T′相MoS 2电化学生成氢
我们报告了密度泛函理论(DFT)计算,以确定单层1T'相MoS 2界面上的析氢反应(HER)的反应途径和势垒和水。通过筛选具有最低质子和电子化学势的界面结构,确定了对预测反应机理很重要的关键结构特征。在HER的典型反应条件下,催化剂表面的质子覆盖率约为ca。当水溶液具有相对低的水合氢盐浓度不超过约37%时,水相的浓度为37%。1.8%。这种对比导致质子通过扩散辅助的Tafel方式而不是先前假定的Heyrovsky方式从催化剂表面解吸。计算出的反应势垒和表面覆盖率与实验估计值的一致性支持了该结果。在预测催化活性时,反应中间体的氢吸附能被广泛用作热力学描述子,而反应障碍通常是动力学参数。我们建议对HER的热力学和动力学描述都应在获得质子和电子最低化学势的前提下进行。
更新日期:2018-07-08
中文翻译:

热力学结构筛选反应机理的单层1T′相MoS 2电化学生成氢
我们报告了密度泛函理论(DFT)计算,以确定单层1T'相MoS 2界面上的析氢反应(HER)的反应途径和势垒和水。通过筛选具有最低质子和电子化学势的界面结构,确定了对预测反应机理很重要的关键结构特征。在HER的典型反应条件下,催化剂表面的质子覆盖率约为ca。当水溶液具有相对低的水合氢盐浓度不超过约37%时,水相的浓度为37%。1.8%。这种对比导致质子通过扩散辅助的Tafel方式而不是先前假定的Heyrovsky方式从催化剂表面解吸。计算出的反应势垒和表面覆盖率与实验估计值的一致性支持了该结果。在预测催化活性时,反应中间体的氢吸附能被广泛用作热力学描述子,而反应障碍通常是动力学参数。我们建议对HER的热力学和动力学描述都应在获得质子和电子最低化学势的前提下进行。


























 京公网安备 11010802027423号
京公网安备 11010802027423号