当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Regiodivergent Synthesis of 1,3‐ and 1,4‐Enynes through Kinetically Favored Hydropalladation and Ligand‐Enforced Carbopalladation
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-07-09 , DOI: 10.1002/anie.201805408 Tapas R. Pradhan 1 , Hong Won Kim 1 , Jin Kyoon Park 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-07-09 , DOI: 10.1002/anie.201805408 Tapas R. Pradhan 1 , Hong Won Kim 1 , Jin Kyoon Park 1
Affiliation
|
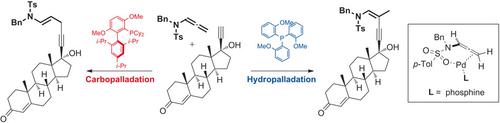
Pd‐catalyzed hydroalkynylations were developed that involve ligand‐enabled regiodivergent addition of an alkyne to an allenamide, giving branched and linear products stereoselectively and facilitated by the neighboring amide group. Regioselectivity was achieved with the use of (o‐OMePh)3P and BrettPhos, which allowed the functionalization of various alkynes, including steroids, carbohydrates, alkaloids, chiral ligands, and vitamins. Based on the experimental results, it was proposed that hydro‐ and carbopalladation processes operated during the formations of the branched and linear products, respectively.
中文翻译:

通过动力学上有利的氢沉和配体增强碳pal的区域发散合成1,3和1,4-炔烃
开发了Pd催化的加氢炔基化反应,涉及将炔烃配体激活的区域发散性加成到烯丙基酰胺上,使支链和线性产物立体选择性地生成,并由相邻的酰胺基团促进。使用(o- OMePh)3 P和BrettPhos可以实现区域选择性,这可以使各种炔烃功能化,包括类固醇,碳水化合物,生物碱,手性配体和维生素。根据实验结果,建议在支链和直链产物的形成过程中分别进行加氢和碳巴巴定过程。
更新日期:2018-07-09
中文翻译:

通过动力学上有利的氢沉和配体增强碳pal的区域发散合成1,3和1,4-炔烃
开发了Pd催化的加氢炔基化反应,涉及将炔烃配体激活的区域发散性加成到烯丙基酰胺上,使支链和线性产物立体选择性地生成,并由相邻的酰胺基团促进。使用(o- OMePh)3 P和BrettPhos可以实现区域选择性,这可以使各种炔烃功能化,包括类固醇,碳水化合物,生物碱,手性配体和维生素。根据实验结果,建议在支链和直链产物的形成过程中分别进行加氢和碳巴巴定过程。


























 京公网安备 11010802027423号
京公网安备 11010802027423号