当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Macromolecular crowding and the importance of proper hydration for the structure and dynamics of protein solutions†
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-07-09 00:00:00 , DOI: 10.1039/c8cp02360c Philipp Honegger 1, 2, 3, 4, 5 , Michael Schmollngruber 1, 2, 3, 4, 5 , Othmar Steinhauser 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-07-09 00:00:00 , DOI: 10.1039/c8cp02360c Philipp Honegger 1, 2, 3, 4, 5 , Michael Schmollngruber 1, 2, 3, 4, 5 , Othmar Steinhauser 1, 2, 3, 4, 5
Affiliation
|
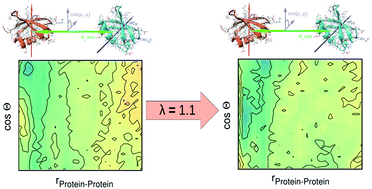
Recent experiments by Weingärtner et al. have given a first hint that dielectric spectroscopy is able to yield a quantitative measure of inter-protein mutual orientation. Therefore, in this computational study, we investigate crowded multi-protein solutions with a special focus on this mutual orientation and its context with dielectric spectroscopy. To the end, existing standard force fields had to be improved by re-scaling the dispersion interaction between protein and water. We find that proper hydration has a strong influence on inter-protein correlations as an enhancement of protein hydration by 10% has a great impact on orientational intermolecular structure. Altogether, the crowding behaviour is improved considerably.
中文翻译:

大分子拥挤以及适当水合作用对蛋白质溶液的结构和动力学的重要性†
Weingärtner等人的最新实验。已经给出了第一个暗示,即介电谱能够对蛋白质间的相互取向进行定量测量。因此,在此计算研究中,我们研究了拥挤的多蛋白溶液,并特别关注了这种相互取向及其介电光谱的背景。最后,必须通过重新缩放蛋白质与水之间的分散相互作用来改善现有的标准力场。我们发现适当的水合作用对蛋白质间的相互关系有很大的影响,因为蛋白质水合作用提高10%对分子间定向结构有很大的影响。总体上,拥挤行为得到了明显改善。
更新日期:2018-07-09
中文翻译:

大分子拥挤以及适当水合作用对蛋白质溶液的结构和动力学的重要性†
Weingärtner等人的最新实验。已经给出了第一个暗示,即介电谱能够对蛋白质间的相互取向进行定量测量。因此,在此计算研究中,我们研究了拥挤的多蛋白溶液,并特别关注了这种相互取向及其介电光谱的背景。最后,必须通过重新缩放蛋白质与水之间的分散相互作用来改善现有的标准力场。我们发现适当的水合作用对蛋白质间的相互关系有很大的影响,因为蛋白质水合作用提高10%对分子间定向结构有很大的影响。总体上,拥挤行为得到了明显改善。


























 京公网安备 11010802027423号
京公网安备 11010802027423号