Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multiform Sulfur Adsorption Centers and Copper-Terminated Active Sites of Nano-CuS for Efficient Elemental Mercury Capture from Coal Combustion Flue Gas
Langmuir ( IF 3.9 ) Pub Date : 2018-07-08 00:00:00 , DOI: 10.1021/acs.langmuir.8b01181 Zequn Yang 1 , Hailong Li 1, 2 , Shihao Feng 2 , Pu Li 1 , Chen Liao 2 , Xi Liu 2 , Jiexia Zhao 2 , Jianping Yang 2 , Po-Heng Lee 3 , Kaimin Shih 1
Langmuir ( IF 3.9 ) Pub Date : 2018-07-08 00:00:00 , DOI: 10.1021/acs.langmuir.8b01181 Zequn Yang 1 , Hailong Li 1, 2 , Shihao Feng 2 , Pu Li 1 , Chen Liao 2 , Xi Liu 2 , Jiexia Zhao 2 , Jianping Yang 2 , Po-Heng Lee 3 , Kaimin Shih 1
Affiliation
|
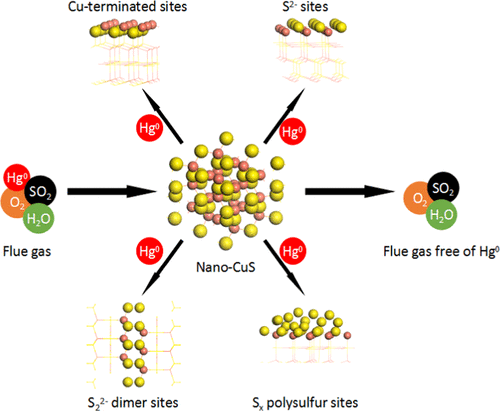
Nanostructured copper sulfide synthesized with the assistance of surfactant with nanoscale particle size and high Brunauer–Emmett–Teller surface area was for the first time applied for the capture of elemental mercury (Hg0) from coal combustion flue gas. The optimal operation temperature of nano-CuS for Hg0 adsorption is 75 °C, which indicates that injection of the sorbent between the wet flue gas desulfurization and the wet electrostatic precipitator systems is feasible. This assures that the sorbent is free of the adverse influence of nitrogen oxides. Oxygen (O2) and sulfur dioxide exerted a slight influence on Hg0 adsorption over the nano-CuS. Water vapor was shown to moderately suppress Hg0 capture efficiency via competitive adsorption. The simulated adsorption capacities of nano-CuS for Hg0 under pure nitrogen (N2), N2 + 4% O2, and simulated flue gas reached 122.40, 112.06, and 89.43 mgHg0/g nano-CuS, respectively. Compared to those of traditional commercial activated carbons and metal sulfides, the simulated adsorption capacities of Hg0 over the nano-CuS are at least an order of magnitude higher. Moreover, with only 5 mg loaded in a fixed-bed reactor, the Hg0 adsorption rate reached 11.93–13.56 μg/g min over nano-CuS. This extremely speedy rate makes nano-CuS promising for a future sorbent injection technique. The anisotropic growth of nano-CuS was confirmed by X-ray diffraction analysis and provided a fundamental aspect for nano-CuS surface reconstruction and polysulfide formation. Further X-ray photoelectron spectroscopy and Hg0 temperature-programmed desorption tests showed that the active polysulfide, S–S dimers, and copper-terminated sites contributed primarily to the extremely high Hg0 adsorption capacity and rate. With these advantages, nano-CuS appears to be a highly promising alternative to traditional sorbents for Hg0 capture from coal combustion flue gas.
中文翻译:

多种形式的硫吸附中心和纳米CuS的铜末端活性位点,可从燃煤烟气中有效捕集元素汞
纳米结构的硫化铜是在具有纳米级粒径和高布鲁诺尔-埃默特-泰勒表面积的表面活性剂的辅助下合成的,首次用于从煤燃烧烟道气中捕集元素汞(Hg 0)。纳米CuS吸附Hg 0的最佳操作温度为75°C,这表明在湿法烟气脱硫和湿法静电除尘器系统之间注入吸附剂是可行的。这确保了吸附剂不受氮氧化物的不利影响。氧(O 2)和二氧化硫对纳米CuS上Hg 0的吸附有轻微影响。显示水蒸气可适度抑制Hg 0通过竞争性吸附来捕获效率。纳米CuS在纯氮(N 2),N 2 + 4%O 2和模拟烟气下对Hg 0的吸附能力分别达到122.40、112.06和89.43 mgHg 0 / g纳米CuS。与传统的商用活性炭和金属硫化物相比,Hg 0在纳米CuS上的模拟吸附容量至少高一个数量级。此外,在固定床反应器中仅装载5 mg的汞,Hg 0纳米CuS的吸附速率达到11.93–13.56μg/ g min。这种极高的速度使纳米CuS有望用于未来的吸附剂注入技术。X射线衍射分析证实了纳米CuS的各向异性生长,并为纳米CuS表面重建和多硫化物的形成提供了基础。进一步的X射线光电子能谱和Hg 0程序升温脱附测试表明,活性多硫化物,S–S二聚体和铜封端的位点主要是对极高的Hg 0吸附能力和速率做出了贡献。凭借这些优势,纳米CuS似乎是从煤燃烧烟气中捕获Hg 0的传统吸附剂的极有希望的替代品。
更新日期:2018-07-08
中文翻译:

多种形式的硫吸附中心和纳米CuS的铜末端活性位点,可从燃煤烟气中有效捕集元素汞
纳米结构的硫化铜是在具有纳米级粒径和高布鲁诺尔-埃默特-泰勒表面积的表面活性剂的辅助下合成的,首次用于从煤燃烧烟道气中捕集元素汞(Hg 0)。纳米CuS吸附Hg 0的最佳操作温度为75°C,这表明在湿法烟气脱硫和湿法静电除尘器系统之间注入吸附剂是可行的。这确保了吸附剂不受氮氧化物的不利影响。氧(O 2)和二氧化硫对纳米CuS上Hg 0的吸附有轻微影响。显示水蒸气可适度抑制Hg 0通过竞争性吸附来捕获效率。纳米CuS在纯氮(N 2),N 2 + 4%O 2和模拟烟气下对Hg 0的吸附能力分别达到122.40、112.06和89.43 mgHg 0 / g纳米CuS。与传统的商用活性炭和金属硫化物相比,Hg 0在纳米CuS上的模拟吸附容量至少高一个数量级。此外,在固定床反应器中仅装载5 mg的汞,Hg 0纳米CuS的吸附速率达到11.93–13.56μg/ g min。这种极高的速度使纳米CuS有望用于未来的吸附剂注入技术。X射线衍射分析证实了纳米CuS的各向异性生长,并为纳米CuS表面重建和多硫化物的形成提供了基础。进一步的X射线光电子能谱和Hg 0程序升温脱附测试表明,活性多硫化物,S–S二聚体和铜封端的位点主要是对极高的Hg 0吸附能力和速率做出了贡献。凭借这些优势,纳米CuS似乎是从煤燃烧烟气中捕获Hg 0的传统吸附剂的极有希望的替代品。


























 京公网安备 11010802027423号
京公网安备 11010802027423号