当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Protein Engineering of the Progesterone Hydroxylating P450‐Monooxygenase CYP17A1 Alters Its Regioselectivity
ChemBioChem ( IF 3.2 ) Pub Date : 2018-08-07 , DOI: 10.1002/cbic.201800371 Lisa K. Morlock 1 , Sascha Grobe 1 , Kathleen Balke 1 , Stephan Mauersberger 2 , Dominique Böttcher 1 , Uwe T. Bornscheuer 1
ChemBioChem ( IF 3.2 ) Pub Date : 2018-08-07 , DOI: 10.1002/cbic.201800371 Lisa K. Morlock 1 , Sascha Grobe 1 , Kathleen Balke 1 , Stephan Mauersberger 2 , Dominique Böttcher 1 , Uwe T. Bornscheuer 1
Affiliation
|
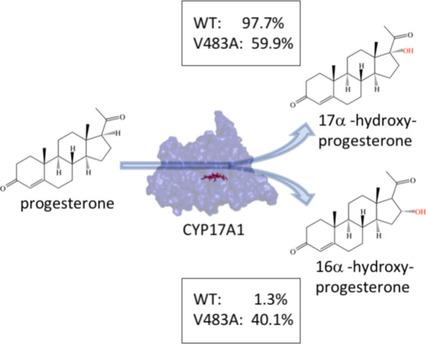
Investigating the active site: Each residue at a distance of 4 Å from the substrate progesterone was replaced by alanine in the active site of the bovine CYP11A1 P450 monooxygenase. This identifies the different residues that are important for overall activity and regioselectivity. Interestingly, a single V483A mutation remarkably changes the regioselectivity to yield 16α‐hydroxyprogesterone.

中文翻译:

孕激素羟化P450-单加氧酶CYP17A1的蛋白质工程改变其区域选择性。
研究活性位点:距底物孕酮4Å处的每个残基在牛CYP11A1 P450单加氧酶的活性位点被丙氨酸替代。这确定了对于总体活性和区域选择性很重要的不同残基。有趣的是,单个V483A突变会显着改变区域选择性,从而产生16α-羟基孕酮。

更新日期:2018-08-07

中文翻译:

孕激素羟化P450-单加氧酶CYP17A1的蛋白质工程改变其区域选择性。
研究活性位点:距底物孕酮4Å处的每个残基在牛CYP11A1 P450单加氧酶的活性位点被丙氨酸替代。这确定了对于总体活性和区域选择性很重要的不同残基。有趣的是,单个V483A突变会显着改变区域选择性,从而产生16α-羟基孕酮。



























 京公网安备 11010802027423号
京公网安备 11010802027423号