当前位置:
X-MOL 学术
›
Adv. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism of Sodium Ion Storage in Na7[H2PV14O42] Anode for Sodium‐Ion Batteries
Advanced Materials Interfaces ( IF 5.4 ) Pub Date : 2018-07-04 , DOI: 10.1002/admi.201800491 Chia-Ching Lin,Wei-Hsiang Lin,Shao-Chu Huang,Chih-Wei Hu,Tsan-Yao Chen,Chi-Ting Hsu,Hao Yang,Ali Haider,Zhengguo Lin,Ulrich Kortz,Ulrich Stimming,Han-Yi Chen
Advanced Materials Interfaces ( IF 5.4 ) Pub Date : 2018-07-04 , DOI: 10.1002/admi.201800491 Chia-Ching Lin,Wei-Hsiang Lin,Shao-Chu Huang,Chih-Wei Hu,Tsan-Yao Chen,Chi-Ting Hsu,Hao Yang,Ali Haider,Zhengguo Lin,Ulrich Kortz,Ulrich Stimming,Han-Yi Chen
|
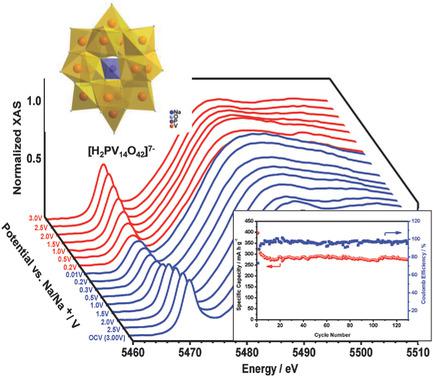
In this work, the authors explore the sodium salt of the 14‐vanado(V)phosphate, Na7[H2PV14O42], as a potential anode material for sodium‐ion batteries (NIBs). The multi‐electron redox activity of the polyoxovanadate [H2PV14O42]7‐leads to high capacity. This polyanion is synthesized by a simple aqueous solution procedure and isolate as a sodium salt with different numbers of crystal waters, Na7[H2PV14O42]·nH2O (n = 15–24). Na7[H2PV14O42] as anode in NIBs exhibits a high and reversible capacity of 322 mA h g−1 at 25 mA g−1 with a high cycling stability (with capacity retention of 87% after 120 cycles). Some of the V5+ ions in [H2PV14O42]7‐ can be reduced to V3+ after being discharged to 0.01 V versus Na/Na+, resulting in an average oxidation state of V3.7+, as based on ex situ X‐ray photoelectron spectroscopy and in situ synchrotron X‐ray absorption near edge structure studies. The crystalline material becomes amorphous during the charge/discharge processes, which can be observed by in situ synchrotron X‐ray diffraction, indicating that functionality does not require crystallinity. The authors propose that the charge storage mechanism of Na7[H2PV14O42] anodes mainly involves redox reactions of V accompanied by insertion/extraction of Na ions in‐between polyoxo‐14‐vanadate ions and adsorption/desorption of Na ions on the surface of the vanadate material.
中文翻译:

Na7 [H2PV14O42]钠离子电池阳极中钠离子的存储机理
在这项工作中,作者探索了14-vanado(V)磷酸盐的钠盐Na 7 [H 2 PV 14 O 42 ],作为钠离子电池(NIB)的潜在阳极材料。聚氧钒酸盐[H 2 PV 14 O 42 ] 7-的多电子氧化还原活性导致高容量。该聚阴离子是通过简单的水溶液法合成的,并作为钠盐与不同数量的结晶水Na 7 [H 2 PV 14 O 42 ]·nH 2 O(n = 15-24)分离。Na 7 [H 2 PV 14O 42 ]作为NIB中的阳极,在25 mA g -1时具有322 mA hg -1的高且可逆容量,并具有高循环稳定性(120个循环后容量保持率达87%)。[H 2 PV 14 O 42 ] 7‐中的一些V 5+离子在放电至相对于Na / Na +的0.01 V电压后可以还原为V 3+,导致平均氧化态为V 3.7+,基于异位X射线光电子能谱和边缘结构附近的原位同步加速器X射线吸收研究。晶体材料在充电/放电过程中变为非晶态,这可以通过原位同步加速器X射线衍射观察到,表明功能不需要结晶性。作者认为,Na 7 [H 2 PV 14 O 42 ]阳极的电荷存储机理主要涉及V的氧化还原反应,伴随着Nax在多氧-14-钒酸盐离子之间的插入/抽出以及Na离子的吸附/解吸。在钒酸盐材料的表面上。
更新日期:2018-07-04
中文翻译:

Na7 [H2PV14O42]钠离子电池阳极中钠离子的存储机理
在这项工作中,作者探索了14-vanado(V)磷酸盐的钠盐Na 7 [H 2 PV 14 O 42 ],作为钠离子电池(NIB)的潜在阳极材料。聚氧钒酸盐[H 2 PV 14 O 42 ] 7-的多电子氧化还原活性导致高容量。该聚阴离子是通过简单的水溶液法合成的,并作为钠盐与不同数量的结晶水Na 7 [H 2 PV 14 O 42 ]·nH 2 O(n = 15-24)分离。Na 7 [H 2 PV 14O 42 ]作为NIB中的阳极,在25 mA g -1时具有322 mA hg -1的高且可逆容量,并具有高循环稳定性(120个循环后容量保持率达87%)。[H 2 PV 14 O 42 ] 7‐中的一些V 5+离子在放电至相对于Na / Na +的0.01 V电压后可以还原为V 3+,导致平均氧化态为V 3.7+,基于异位X射线光电子能谱和边缘结构附近的原位同步加速器X射线吸收研究。晶体材料在充电/放电过程中变为非晶态,这可以通过原位同步加速器X射线衍射观察到,表明功能不需要结晶性。作者认为,Na 7 [H 2 PV 14 O 42 ]阳极的电荷存储机理主要涉及V的氧化还原反应,伴随着Nax在多氧-14-钒酸盐离子之间的插入/抽出以及Na离子的吸附/解吸。在钒酸盐材料的表面上。


























 京公网安备 11010802027423号
京公网安备 11010802027423号