当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fully Dispersed Rh Ensemble Catalyst to Enhance Low-Temperature Activity
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-07-05 , DOI: 10.1021/jacs.8b04613 Hojin Jeong 1 , Geonhee Lee 2 , Beom-Sik Kim 1 , Junemin Bae 1 , Jeong Woo Han 3 , Hyunjoo Lee 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-07-05 , DOI: 10.1021/jacs.8b04613 Hojin Jeong 1 , Geonhee Lee 2 , Beom-Sik Kim 1 , Junemin Bae 1 , Jeong Woo Han 3 , Hyunjoo Lee 1
Affiliation
|
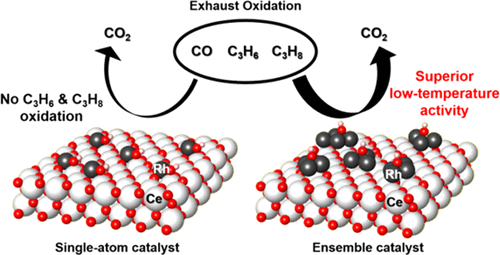
Minimizing the use of precious metal catalysts is important in many applications. Single-atom catalysts (SACs) have received much attention because all of the metal atoms can be used for surface reactions. However, SACs cannot catalyze some important reactions that require ensemble sites. Here, Rh catalysts were prepared by treating 2 wt % Rh/CeO2 hydrothermally at 750 °C for 25 h. Nearly 100% dispersion was obtained, but the surface Rh atoms were not isolated (denoted as ENS). They catalyzed the oxidation of C3H6 or C3H8 at low temperatures, but these oxidations did not occur on the Rh SAC. When the simultaneous oxidation of CO, C3H6, and C3H8 was performed, the T20 (temperature at conversion 20%) for CO oxidation increased significantly from 40 °C for sole CO oxidation to 180 °C on SAC due to the competitive adsorption of hydrocarbons. However, T20 increased much less on ENS, from 60 to 100 °C. ENS exhibited superior activity for low-temperature oxidation. During hydrothermal treatment for 25 h, the Rh size initially increased from 2.3 to 6.7 nm then decreased to 0.9 nm. The surface hydroxyl groups formed on the catalyst surface help detach Rh atoms from Rh clusters, while preventing the reaggregation of dispersed Rh atoms into Rh clusters. This fully dispersed catalyst would have maximum atom-efficiency while catalyzing various surface reactions.
中文翻译:

用于增强低温活性的完全分散的 Rh 集合催化剂
在许多应用中,尽量减少贵金属催化剂的使用很重要。单原子催化剂 (SAC) 受到了很多关注,因为所有金属原子都可以用于表面反应。然而,SACs 不能催化一些需要集合位点的重要反应。在此,Rh 催化剂是通过在 750 °C 下水热处理 2 wt% Rh/CeO2 25 小时来制备的。获得了接近 100% 的分散,但表面的 Rh 原子没有被隔离(表示为 ENS)。它们在低温下催化了 C3H6 或 C3H8 的氧化,但这些氧化并未发生在 Rh SAC 上。当同时进行 CO、C3H6 和 C3H8 氧化时,由于碳氢化合物的竞争吸附,CO 氧化的 T20(转化率 20% 时的温度)从单独 CO 氧化的 40°C 显着增加到 SAC 上的 180°C。然而,T20 在 ENS 上的增加要少得多,从 60°C 到 100°C。ENS 表现出优异的低温氧化活性。在水热处理 25 小时期间,Rh 尺寸最初从 2.3 增加到 6.7 nm,然后减少到 0.9 nm。在催化剂表面形成的表面羟基有助于将 Rh 原子从 Rh 簇中分离,同时防止分散的 Rh 原子重新聚集成 Rh 簇。这种完全分散的催化剂在催化各种表面反应时将具有最大的原子效率。同时防止分散的 Rh 原子重新聚集成 Rh 簇。这种完全分散的催化剂在催化各种表面反应时将具有最大的原子效率。同时防止分散的 Rh 原子重新聚集成 Rh 簇。这种完全分散的催化剂在催化各种表面反应时将具有最大的原子效率。
更新日期:2018-07-05
中文翻译:

用于增强低温活性的完全分散的 Rh 集合催化剂
在许多应用中,尽量减少贵金属催化剂的使用很重要。单原子催化剂 (SAC) 受到了很多关注,因为所有金属原子都可以用于表面反应。然而,SACs 不能催化一些需要集合位点的重要反应。在此,Rh 催化剂是通过在 750 °C 下水热处理 2 wt% Rh/CeO2 25 小时来制备的。获得了接近 100% 的分散,但表面的 Rh 原子没有被隔离(表示为 ENS)。它们在低温下催化了 C3H6 或 C3H8 的氧化,但这些氧化并未发生在 Rh SAC 上。当同时进行 CO、C3H6 和 C3H8 氧化时,由于碳氢化合物的竞争吸附,CO 氧化的 T20(转化率 20% 时的温度)从单独 CO 氧化的 40°C 显着增加到 SAC 上的 180°C。然而,T20 在 ENS 上的增加要少得多,从 60°C 到 100°C。ENS 表现出优异的低温氧化活性。在水热处理 25 小时期间,Rh 尺寸最初从 2.3 增加到 6.7 nm,然后减少到 0.9 nm。在催化剂表面形成的表面羟基有助于将 Rh 原子从 Rh 簇中分离,同时防止分散的 Rh 原子重新聚集成 Rh 簇。这种完全分散的催化剂在催化各种表面反应时将具有最大的原子效率。同时防止分散的 Rh 原子重新聚集成 Rh 簇。这种完全分散的催化剂在催化各种表面反应时将具有最大的原子效率。同时防止分散的 Rh 原子重新聚集成 Rh 簇。这种完全分散的催化剂在催化各种表面反应时将具有最大的原子效率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号