当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modular Assembly of a Conserved Repetitive Sequence in the Spider Eggcase Silk: From Gene to Fiber
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acsbiomaterials.8b00428 Jianming Chen 1 , Jinlian Hu 1 , Sono Sasaki 2 , Kensuke Naka 3
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acsbiomaterials.8b00428 Jianming Chen 1 , Jinlian Hu 1 , Sono Sasaki 2 , Kensuke Naka 3
Affiliation
|
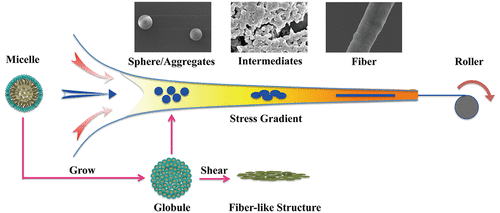
Spider silk features extraordinary toughness in combination with great biocompatibility and biodegradability, fascinating researchers to prepare artificial silk fibers inspired from the natural art of spinning. In addition to C- and N-terminal domain, a repeat unit from Lactrodectus mactans spider eggcase silks displays substantial sequence conservation across species. Herein, we attempt to spin the engineered tubuliform spidroin 1 (eTuSp1) by microfluidics in a mode of modular assembly comprising the genetic construction, micellar formation, phase separation, and further solidification. Based on the conserved gene sequence, a unique amphiphilic behavior was predicted and then verified by combined techniques of dynamic light scattering, transmission electron microscopy, and synchrotron radiation X-ray diffraction to reveal the formation of micelle-like structure. Through the employ of biomimetic microfluidic devices, desolvation of eTuSp1 was simplified by the nonsolvent induced phase separation in place of the conventional ions exchange and acidification. Both controlled by protein concentrations and flow rate ratios, silk fibers were assembled similar to these reported in other studies of spheres/spherical aggregates observed as intermediates. Because of the applied shear and elongational flow in microfluidic systems, these intermediates were forced to form fibrillar assemblies accompanied by the conformational transformation from α-helix to β-sheet. The resultant mechanical properties were investigated in response to the change of secondary structures and morphologies during spinning process. This work studies the sequence–structure–property relationship, providing comprehensive and systematic insight into the design rational on the preparation of artificial silk fibers from microscale to macroscale.
中文翻译:

蜘蛛蛋壳丝中保守重复序列的模块组装:从基因到纤维
蜘蛛丝具有非凡的韧性,并具有出色的生物相容性和生物降解性,使研究人员着迷于从纺丝自然艺术中汲取灵感的人造丝纤维的制备。除C和N端结构域外,Mactac的一个重复单元蜘蛛蛋壳丝显示出跨物种的实质性序列保守性。在本文中,我们尝试通过微流控技术以一种包括遗传构建,胶束形成,相分离和进一步固化的模块化组装方式旋转工程化的管状微孢子蛋白1(eTuSp1)。基于保守的基因序列,预测了独特的两亲性行为,然后通过动态光散射,透射电子显微镜和同步辐射X射线衍射技术相结合,证实了胶束状结构的形成。通过使用仿生微流体装置,通过非溶剂诱导的相分离代替了常规的离子交换和酸化,简化了eTuSp1的去溶剂化。两者都受蛋白质浓度和流速比的控制,蚕丝纤维的组装类似于其他关于作为中间体的球体/球形聚集体的研究报告。由于在微流体系统中施加了剪切力和伸长流,这些中间体被迫形成纤维状组件,并伴随着从α-螺旋到β-片层的构象转变。响应于纺丝过程中二级结构和形态的变化,对所得的机械性能进行了研究。这项工作研究了序列-结构-属性的关系,为从微观到宏观的人造丝纤维制备的合理设计提供了全面而系统的见解。由于在微流体系统中施加了剪切力和伸长流,这些中间体被迫形成纤维状组件,并伴随着从α-螺旋到β-片层的构象转变。响应于纺丝过程中二级结构和形态的变化,对所得的机械性能进行了研究。这项工作研究了序列-结构-属性的关系,为从微观到宏观的人造丝纤维制备的合理设计提供了全面而系统的见解。由于在微流体系统中施加了剪切力和伸长流,这些中间体被迫形成纤维状组件,并伴随着从α-螺旋到β-片层的构象转变。响应于纺丝过程中二级结构和形态的变化,对所得的机械性能进行了研究。这项工作研究了序列-结构-属性的关系,为从微观到宏观的人造丝纤维制备的合理设计提供了全面而系统的见解。响应于纺丝过程中二级结构和形态的变化,对所得的机械性能进行了研究。这项工作研究了序列-结构-属性的关系,为从微观到宏观的人造丝纤维制备的合理设计提供了全面而系统的见解。响应于纺丝过程中二级结构和形态的变化,对所得的机械性能进行了研究。这项工作研究了序列-结构-属性的关系,为从微观到宏观的人造丝纤维制备的合理设计提供了全面而系统的见解。
更新日期:2018-07-06
中文翻译:

蜘蛛蛋壳丝中保守重复序列的模块组装:从基因到纤维
蜘蛛丝具有非凡的韧性,并具有出色的生物相容性和生物降解性,使研究人员着迷于从纺丝自然艺术中汲取灵感的人造丝纤维的制备。除C和N端结构域外,Mactac的一个重复单元蜘蛛蛋壳丝显示出跨物种的实质性序列保守性。在本文中,我们尝试通过微流控技术以一种包括遗传构建,胶束形成,相分离和进一步固化的模块化组装方式旋转工程化的管状微孢子蛋白1(eTuSp1)。基于保守的基因序列,预测了独特的两亲性行为,然后通过动态光散射,透射电子显微镜和同步辐射X射线衍射技术相结合,证实了胶束状结构的形成。通过使用仿生微流体装置,通过非溶剂诱导的相分离代替了常规的离子交换和酸化,简化了eTuSp1的去溶剂化。两者都受蛋白质浓度和流速比的控制,蚕丝纤维的组装类似于其他关于作为中间体的球体/球形聚集体的研究报告。由于在微流体系统中施加了剪切力和伸长流,这些中间体被迫形成纤维状组件,并伴随着从α-螺旋到β-片层的构象转变。响应于纺丝过程中二级结构和形态的变化,对所得的机械性能进行了研究。这项工作研究了序列-结构-属性的关系,为从微观到宏观的人造丝纤维制备的合理设计提供了全面而系统的见解。由于在微流体系统中施加了剪切力和伸长流,这些中间体被迫形成纤维状组件,并伴随着从α-螺旋到β-片层的构象转变。响应于纺丝过程中二级结构和形态的变化,对所得的机械性能进行了研究。这项工作研究了序列-结构-属性的关系,为从微观到宏观的人造丝纤维制备的合理设计提供了全面而系统的见解。由于在微流体系统中施加了剪切力和伸长流,这些中间体被迫形成纤维状组件,并伴随着从α-螺旋到β-片层的构象转变。响应于纺丝过程中二级结构和形态的变化,对所得的机械性能进行了研究。这项工作研究了序列-结构-属性的关系,为从微观到宏观的人造丝纤维制备的合理设计提供了全面而系统的见解。响应于纺丝过程中二级结构和形态的变化,对所得的机械性能进行了研究。这项工作研究了序列-结构-属性的关系,为从微观到宏观的人造丝纤维制备的合理设计提供了全面而系统的见解。响应于纺丝过程中二级结构和形态的变化,对所得的机械性能进行了研究。这项工作研究了序列-结构-属性的关系,为从微观到宏观的人造丝纤维制备的合理设计提供了全面而系统的见解。

























 京公网安备 11010802027423号
京公网安备 11010802027423号