当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient syntheses and anti-cancer activity of xenortides A–D including ent/epi-stereoisomers†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-07-05 00:00:00 , DOI: 10.1039/c8ob00452h N. Esmati 1, 2, 3, 4 , A. R. Maddirala 1, 2, 3, 4 , N. Hussein 4, 5, 6, 7 , H. Amawi 4, 5, 6, 7 , A. K. Tiwari 4, 5, 6, 7 , P. R. Andreana 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-07-05 00:00:00 , DOI: 10.1039/c8ob00452h N. Esmati 1, 2, 3, 4 , A. R. Maddirala 1, 2, 3, 4 , N. Hussein 4, 5, 6, 7 , H. Amawi 4, 5, 6, 7 , A. K. Tiwari 4, 5, 6, 7 , P. R. Andreana 1, 2, 3, 4
Affiliation
|
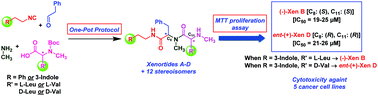
A one-pot, two-step, total synthesis of naturally occurring xenortides A, B, C and D, (Xens A–D) isolated from the bacterium Xenorhabdus nematophila, and an entire complementary set of stereoisomers, has been achieved. Compounds were synthesized utilizing an isocyanide-based Ugi 4-CR followed by facile N-Boc deprotection. The reaction sequence took advantage of the chiral pool of N-Boc protected amino acids (L-Leu/Val and D-Leu/Val) with aryl isocyanides, phenyl acetaldehyde and methylamine giving the desired Xens A–D (A and B >98% ee) and all subsequent stereoisomers in reasonable yields upon deprotection followed by separation of diastereomers. Also, detailed mechanistic insights for diastereoselectivity of (−)-Xen A, as a model in the Ugi 4-CR, has been described. Moreover, for the first time, this focused library was screened for cytotoxicity against a panel of epithelial cancer cell lines as well as normal cell lines with an MTT proliferation assay. The structure–activity relationship (SAR) study demonstrated that tryptamides Xen B and D were more active than phenylethylamides Xen A and C. Furthermore, (−)-Xen B (IC50 = 19–25 μM) and ent-(+)-Xen D (IC50 = 21–26 μM) gave the highest cytotoxicity and they were also found to be non-toxic toward normal cells. Importantly, the SAR results indicate that the stereochemistry at C8 and C11 in (−)-Xen B and ent-(+)-Xen D play a critical role in cytotoxic activity.
中文翻译:

Xenortides A–D的有效合成和抗癌活性,包括ent / epi-立体异构体†
一锅,两步,从细菌线虫Xenorhabdus nematophila细菌分离得到的天然存在的Xenortides A,B,C和D(Xens A–D)和一套完整的立体异构体完全合成。利用基于异氰酸酯的Ugi 4-CR进行合成,然后进行简单的N -Boc脱保护反应合成化合物。反应序列利用了N -Boc保护的氨基酸的手性库(L -Leu / Val和D-Leu / Val)与芳基异氰化物,苯乙醛和甲胺一起得到所需的Xens A–D(A和B> 98%ee),并在脱保护后以合理的收率分离所有随后的立体异构体,然后分离非对映异构体。而且,已经描述了作为Ugi 4-CR模型的(-)-Xen A的非对映选择性的详细机械原理。此外,首次使用MTT增殖试验筛选了该聚焦文库对一组上皮癌细胞系和正常细胞系的细胞毒性。结构-活性关系(SAR)研究表明,色胺Xen B和D比苯乙酰胺Xen A和C更具活性。此外,(-)-Xen B(IC 50 = 19–25μM)和ent -(+)- Xen D(IC 50= 21–26μM)具有最高的细胞毒性,并且它们也对正常细胞无毒。重要的是,SAR结果表明(-)-Xen B和ent -(+)-Xen D中C 8和C 11的立体化学在细胞毒性活性中起关键作用。
更新日期:2018-07-05
中文翻译:

Xenortides A–D的有效合成和抗癌活性,包括ent / epi-立体异构体†
一锅,两步,从细菌线虫Xenorhabdus nematophila细菌分离得到的天然存在的Xenortides A,B,C和D(Xens A–D)和一套完整的立体异构体完全合成。利用基于异氰酸酯的Ugi 4-CR进行合成,然后进行简单的N -Boc脱保护反应合成化合物。反应序列利用了N -Boc保护的氨基酸的手性库(L -Leu / Val和D-Leu / Val)与芳基异氰化物,苯乙醛和甲胺一起得到所需的Xens A–D(A和B> 98%ee),并在脱保护后以合理的收率分离所有随后的立体异构体,然后分离非对映异构体。而且,已经描述了作为Ugi 4-CR模型的(-)-Xen A的非对映选择性的详细机械原理。此外,首次使用MTT增殖试验筛选了该聚焦文库对一组上皮癌细胞系和正常细胞系的细胞毒性。结构-活性关系(SAR)研究表明,色胺Xen B和D比苯乙酰胺Xen A和C更具活性。此外,(-)-Xen B(IC 50 = 19–25μM)和ent -(+)- Xen D(IC 50= 21–26μM)具有最高的细胞毒性,并且它们也对正常细胞无毒。重要的是,SAR结果表明(-)-Xen B和ent -(+)-Xen D中C 8和C 11的立体化学在细胞毒性活性中起关键作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号